Abstract
The sweet potato weevil, Cylas formicarius is the most important pest of sweet potato, Ipomoea batatas (L.). In this study, we have sequenced the complete mitochondrial genome of Cylas formicarius (Coleoptera: Brentidae). The mitochondrial genome is 17,150 bp length (GenBank no. MK421358) has A + T content of 78.4% (A = 39.3%; T = 39.1%; C = 13.2%, and G = 8.4%), which is the classical structure for insect mitogenome. All PCGs started with ATN except COX2, which started with TTG and all PCGs terminated with conventional stop codons TAA/TAG. The phylogenetic tree confirms that C. formicarius is clustered with other three Brentidae with strongly supported. This study enriches the mitogenomes of the Coleoptera family Brentidae.
The sweet potato weevil, Cylas formicarius (F.) (Coleoptera: Brentidae), is the most important pest of sweetpotato, Ipomoea batatas (L.), throughout the tropics and subtropics (Talekar Citation1982; Chalfant et al. Citation1990). The weevil completes its life cycle within either the tuber or vine of the sweetpotato plant and they attack sweet potatoes both in the field and in storage (Hansen et al. Citation1992). Sutherland (Citation1986) reported that C. formicarius damage lead to yield losses ranging from 5 to 80% and feeding by C. formicarius elicits terpenoid production in sweet potato storage tubers that results in damage, unpalatable tubers (Uritani et al. Citation1975). In this study, we have sequenced and annotated the complete mitochondrial genome (mitogenome) of C. formicarius by next-generation sequencing method for the first time, which is obtained for weevils in this study and will facilitate future studies on the identification, population genetics, and evolution of weevil subfamily Cyladinae.
Total genome DNA was extracted from a male adult of C. formicarius which was collected from sweet potato, in Guizhou Province, China, in September 2018. Also, voucher specimen’s genome DNA and male external genitalia are deposited in the Institute of Entomology, Guizhou University, Guiyang, China (GUGC). The complete mitogenome of C. formicarius is 17,150 bp length (GenBank no. MK421358), which contains 37 genes (22 tRNA genes, 13 protein-coding genes (PCGs), and a control region) and we have found two large intergenic spacers (IGSs). IGS1 is located between trn-I and trn-Q with 964 bp; IGS2 is located between ten-S2 and nad1, which is similar with Meloidae’s previous studies (Du et al. Citation2017). In general, the C. formicarius mitogenome has an A + T content of 78.4% (A = 39.3%; T = 39.1%; C = 13.2%, and G = 8.4%). All PCGs started with ATN except COX2, which started with TTG and all PCGs terminated with conventional stop codons TAA/TAG. All tRNA genes are identified by ARWEN version 1.2 software (Laslett and Canbäck Citation2008). The 16S rRNA gene is 1297 bp in size and is located between trn-L2 and trn-V; the 12S rRNA gene is 767 bp in length and is located after trn-V. The control region is 1317 bp long and is located between 12S rRNA and trn-I.
The phylogenetic relationships of C. formicarius were reconstructed using MrBayes analysis based upon the concatenated nucleotide sequences of the 13 PCGs (). The 13 PCG sequences without stop codons were used in the phylogenetic analysis. Each PCG was aligned individually with codon-based multiple alignments using the MAFFT algorithm in the TranslatorX online server (Abascal et al. Citation2010). Phylogenetic trees were generated using MrBayes software (MrBayes 3.1.2) (Miller et al. Citation2010) with the GTR + I + G model.
Figure 1. Phylogenetic analyses of C. formicarius based upon the concatenated nucleotide sequences of the 13 PCGs of 27 species. The analysis was performed using MrBayes software. Numbers at nodes are posterior probability. The accession number for each species is indicated after the scientific name.
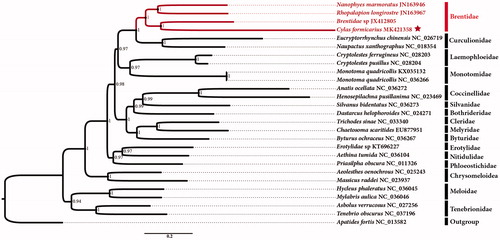
The phylogenetic tree confirms that C. formicarius is clustered with other three Brentidae with strongly supported (posterior probability = 1); Brentidae and Curculionidae are sister group in this study. Until now, only three partial mitogenomes of Brentidae have been sequenced, and we hope that our data can be useful for further study.
Disclosure statement
The authors claim no conflict of interest.
Additional information
Funding
References
- Abascal F, Zardoya R, Telford MJ. 2010. TranslatorX: multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 38:W7–W13.
- Chalfant RB, Jansson RK, Seal DR, Schalk JM. 1990. Ecology and management of sweet potato insects. Annu Rev Entomol. 35:157–180.
- Du C, Zhang L, Lu T, Ma J, Zeng C, Yue B, Zhang X. 2017. Mitochondrial genomes of blister beetles (Coleoptera, Meloidae) and two large intergenic spacers in Hycleus genera. BMC Genomics. 18:698. [CrossRef]1
- Hansen JD, Emerson CL, Signorotti DA. 1992. Visual detection of sweetpotato weevil by non-invasive methods. Fla Entomol. 75:369–375.
- Laslett D, Canbäck B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24: 172–175.
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE); Nov 14, 2010; New Orleans, LA; p. 1–8.1
- Sutherland JA. 1986. A review of the biology and control of the sweetpotato weevil Cylas formicarius (Fab). Tropical Pest Manage. 32:304–305.
- Talekar NS. 1982. Effects of a sweetpotato weevil (Coleoptera: Curculionidae) infestation on sweet potato root yields. J Econ Entomol. 75:1042–1044.
- Uritani I, Saito T, Honda H. 1975. Induction of furano-terpenoids in sweet potato roots by the larval components of the sweet potato weevils. Agric Biol Chem. 39:1857–1862.
