Abstract
Tomato (Solanum lycopersicum L.) is native to Peru and Mexico. It is an important vegetable crop of the world, which ranks next to potato in importance. In addition, tomato is one of the most widely cultivated fruit and vegetables in the world. In this study, we obtained the complete chloroplast genome sequence of Tomato using BGISEQ-500 sequencing. Its chloroplast genome size has 155,474 bp, containing a large single copy region (85,856 bp), a small single copy region (18,374 bp), and a pair of IR regions (25,622 bp). The overall GC contents of the chloroplast genome were 38.0%. This circular genome contains 142 annotated genes, including 89 protein-coding genes, 45 tRNAs and 8 rRNAs. The phylogenetic relationship analysis using Maximum Likelihood method showed that tomato (Solanum lycopersicum L.) much closely related to two kinds of wild tomatoes Solanum pimpinellifolium and Solanum cheesmaniae. This complete chloroplast genomes can be subsequently used for the genetic breeding and useful to study the evolutionary processes in Lycopersicon.
Tomato (Solanum lycopersicum L.) is the most important crop plant and a model system for fruit plant. In addition, tomato is one of the largest angiosperm genera in the fruits and vegetables (Consortium Citation2012). Tomato has a variety of vitamins and nutrients, such as rich in Vitamin C and A, folic acid, potassium, and others. In particular, tomato contains lycopene, which is more beneficial to human health (Dorais et al. Citation2008). Worldwide tomato yield and trade in tomato products are growing rapidly, and China’s tomato yield and trade are growing an increasingly important role in the world tomato trade, which will have an important influence on the world tomato trade in the future. Genomics and modern biotechnology can be used to study the molecular mechanisms of tomato lycopene synthesis and tomato molecular breeding. In this study, we obtained the complete chloroplast genome of Solanum lycopersicum L. and explored the phylogenetic relationship with other species, which can be subsequently used for the tomato genetic breeding and study the evolution in Lycopersicon.
The specimen of Solanum lycopersicum L. was isolated from College of Horticulture, Jilin Agricultural University test field in Jilin, Changchun, China (43.82N, 125.41E). The total genomic DNA of Solanum lycopersicum L. was extracted using Plant Genomic DNA Kit (TIANGEN BIOTRCH, Beijing, CA, CHN) and stored in Jilin Agricultural University College of Horticulture (No. JAUCH02). The whole genomic DNA sequencing was performed by the BGISEQ-500 Sequencing Platform (BIG, Shenzhen, CA, CHN). The chloroplast genome was assembled with SPAdes Version 3.9 (Bankevich et al. Citation2012) and annotated by Blast and DOGMA (Wyman et al. Citation2004). The tRNA genes were further identified using tRNAscan-SE Version 2.0 (Lowe and Chan Citation2016). The annotated chloroplast genome was submitted to GenBank database under accession No. MK0434521.
The complete chloroplast genome of Solanum lycopersicum L. was a circle with 155,474 bp in size, containing a large single-copy region of 85,856 bp, a small single-copy region of 18,374 bp and a pair of inverted repeat regions (IRs) of 25,622 bp. In total 142 genes were annotated on the tomato chloroplast genome, including 89 protein-coding genes (PCG), 45 transfer RNA genes (tRNA), and 8 ribosomal RNA genes (rRNA). In the IR regions, a total of 18 genes were found duplicated, including 5 PCG species (rps19, rpl2, rpl23, rps7, and rps12), 9 tRNA species (trnI-CAU, trnL-CAA, trnV-GAC, trnI-GAU, trnI-GAU, trnA-UGC, trnA-UGC, trnR-ACG, and trnN-GUU), and 4 rRNA species (rrn16, rrn23, rrn4.5, and rrn5). The overall nucleotide composition is 30.6% A, 31.4% T, 19.2% C, and 18.8% G, with a total G + C content of 38.0%.
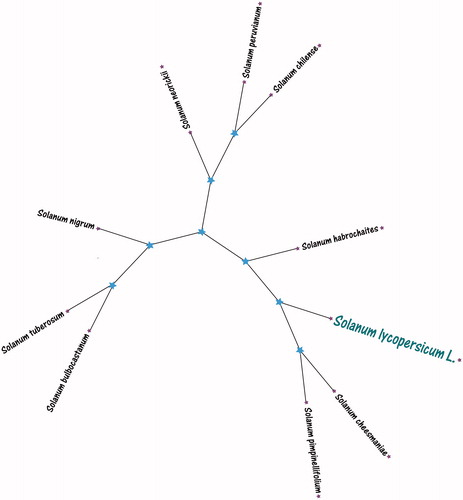
For phylogenetic relationship analysis, we selected tomato (Solanum lycopersicum L.) with other nine species chloroplast genomes from GenBank to assess the relationship of them. The genome-wide alignment of all chloroplast genomes was constructed by HomBlocks (Bi et al. Citation2018). The phylogenetic trees were reconstructed using Maximum Likelihood (ML) methods. ML analysis was performed using RAxML Version 8.2.4 (Stamatakis Citation2014), of which the bootstrap values were calculated using 5000 replicates to assess node support and all the nodes were inferred with strong support by the ML methods. The final tree was represented and edited using MEGA Version X (Kumar et al. Citation2018). As shown in the phylogenetic tree (), the chloroplast genome of showed that tomato (Solanum lycopersicum L.) was the closest and clustered with two kinds of wild tomatoes Solanum pimpinellifolium and Solanum cheesmaniae.
Figure 1. ML tree of Tomato (Solanum lycopersicum L.) with other nine species chloroplast genomes. This tree was drawn without setting out groups. All nodes exhibit above 90% bootstraps. The NCBI database accession number is Solanum habrochaites (KP117023.1), Solanum pimpinellifolium (KP117027.1), Solanum cheesmaniae (KP117020.1), Solanum chilense (KP117021.1), Solanum neorickii (KP117025.1), Solanum peruvianum (KP117026.1), Solanum tuberosum (KM489056.2), Solanum bulbocastanum (NC007943.1), Solanum nigrum (DQ347958.1).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19:455–477.
- Bi G, Mao Y, Xing Q, Cao M. 2018. HomBlocks: a multiple-alignment construction pipeline for organelle phylogenomics based on locally collinear block searching. Genomics. 110:18–22.
- Consortium TTG. 2012. The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 485:635.
- Dorais M, Ehret DL, Papadopoulos AP. 2008. Tomato (Solanum lycopersicum) health components: from the seed to the consumer. Phytochem Rev. 7:231–250.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35:1547–1549.
- Lowe TM, Chan PP. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 44:54–57.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.
