Abstract
Hippophae neurocarpa are dioecious deciduous shrubs, diffused in the Qinghai–Tibetan Plateau at high altitude. Here, we report the complete chloroplast genome of H. neurocarpa. The chloroplast genome is found to be 156,316 bp in length with 36.65% GC contents. The chloroplast genome sequences contained 124 genes, including 78 protein-coding genes, 38 tRNA genes, and 8 rRNA genes. The complete chloroplast genome of H. neurocarpa will be beneficial for identifying molecular markers for further conservation and utilization of these multipurpose natural resources.
Hippophae neurocarpa S. W. Liu et T. N. He, are dioecious deciduous shrubs that belong to the family Elaeagnaceae in the genus Hippophae (Lian Citation2000). Topologically effected by the Quaternary climatic oscillation, natural H. neurocarpa are only diffused in the Qinghai–Tibetan Plateau at high altitude (Meng et al. Citation2008). The H. neurocarpa berry juice is rich in vitamins (A, C, E, K, and P), which functions as health food and medicine (Ruan et al. Citation2000).
Hippophae neurocarpa have diploid and polymorphic chromosomes, including one pair sex-chromosomes (Elena et al. Citation2011). Like most dioecious perennials, neither male nor female H. neurocarpa can be distinguished until the berries ripe, which needs 5–7 years. Incomes will rise if pistillate trees take major ratio on plantations, with only 6–7% male genotypes supplying qualified sperms (Walf and Wegart Citation1993). However, no molecular markers have been developed to determine gender at the juvenile stage, and no complete chloroplast genome of H. neurocarpa has been released.
In this study, we report and characterize the complete chloroplast genomes of H. neurocarpa. Young and fresh leaves were collected from a plantation in Qinghai Province, China (N37°01′55.57″, E101°33′12.67″), and the specimens were deposited in the Qinghai–Tibetan Plateau Museum of Biology (HNWP). Genomic DNA was extracted following the modified CTAB method (Doyle Citation1987). The complete chloroplast genome was sequenced using an Illumina HiSeq4000 platform, assembled with SPAdes version 3.9.1 (Bankevich et al. Citation2012) and annotated with CpGAVAS (Liu et al. Citation2012). Phylogenies trees were generated by maximum-likelihood (ML) analysis based on concatenated 30 complete cp genomes. The sequences were initially aligned using MAFFT, with the maximum-likelihood (ML) inference with 100 bootstrap replicates by RaxML-HPC2 on TG ver. 7.2.8 on the Cipres web server (Stamatakis et al. Citation2008). A General Time Reversible + Proportion Invariation + Gamma nucleotide substitution model (GTR + G + I) was selected as the best substitution model for the ML and BI analyses. BI analyses were conducted using Mrbayes v 3.2.6 (Ronquist and Huelsenbeck Citation2003).
The complete chloroplast genome of H. neurocarpa has a total length of 156,316 bp (MK787303). The typical quadripartite structure consisted of a pair of IRs of 26,653 bp, an LSC region of 83,968 bp, an SSC region of 19,042 bp, respectively. The GC content of the chloroplast genomes was 36.65%. There were 124 predicted genes, 78 protein-coding genes, 38 tRNA genes, and 8 rRNA genes.
A total of 73 Simple Sequence Repeats (SSRs) or microsatellites were identified, and there were 68, 4, and 1 mono-, di-, and tri-nucleotide repeats, respectively. Mononucleotide SSRs were the richest (93.15%). All the protein-coding genes presented a total of 26,424 codons, with leucine (2775 codons, ∼10.50% of the total) being the most abundant amino acid.
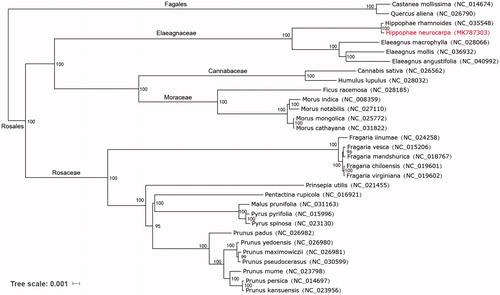
ML analysis showed that H. neurocarpa first formed a small branch with H. rhamnoides, and then formed a single branch with Elaeagnaceae family plants (). The complete chloroplast genome of H. neurocarpa can provide a reference for developing markers for further studies on the phylogeny and evolution of the genus Hippophae plants, even conservation and utilization of these multipurpose natural resources.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19:455–477.
- Doyle JJ. 1987. A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Elena T, Capraru G, Rosu CM, Zamfirache MM, Olteanu Z, Manzu C. 2011. Morphometric pattern of somatic chromosomes in three Romanian seabuckthorn genotypes. Caryologia. 64:189–196.
- Lian YS. 2000. The plant biology and chemistry of Hippophaë.Lanzhou. Lanzhou, Gansu: Gansu Science and Technology Press; p. 36–41.
- Liu C, Shi L, Zhu Y, Chen H, Zhang J, Lin X, Guan X. 2012. CpGAVAS, an integrated web server for the annotation, visualization, analysis, and genbank submission of completely sequenced chloroplast genome sequences. BMC Genomics. 13:715.
- Meng LH, Yang HL, Wu GL, Wang YJ. 2008. Phylogeography of Hippophae neurocarpa (Elaeagnaceae) inferred from the chloroplast DNA trnL-F sequence variation. J System Evol. 46:32–40.
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19:1572–1574.
- Ruan CJ, Xie QL, Li DQ. 2000. Function and benefits of sea buckthorn improving eco environment of loess plateaus. Environ Prot. 5:30–31.
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 57:758–771.
- Walf D, Wegart F. 1993. Cultivation and utilization of wild fruit crops. Braunschweig, Germany: Bernhard Thalacker Veriage Gmbh & Co. Experience gained in the harvesting and utilization of sea buckthorn; p. 22–29.