Abstract
A 16,359 bp mitochondrial genome of Laodelphax striatellus collected in a southern part of Korean peninsula was completed and their intraspecies variations were compared with Korean and Chinese L. striatellus mitogenomes. The circular mitogenome of L. striatellus contains 13 protein-coding genes, two rRNA genes, 22 tRNAs, and a single large non-coding region of 1,972 bp. The base composition was AT-biased (77.3%). It has only one single nucleotide polymorphism (SNP) in AT-rich region compared to other Korean mitogenome, but total 41 and 141 SNPs and 118 and 166 insertions and deletions, respectively, compared to two Chinese mitogenomes, suggesting the possibility of tracing migration based on geographic genetic diversity.
Laodelphax striatellus (Fallén, 1826), called as small brown planthopper (SBPH), is distributed in Asia, Europe, and Northern Africa (Wilson and Claridge Citation1991). It is one of the important pests in East Asia where rice has been cultivated intensively (Hibino Citation1996; Seo et al. Citation2016). Long-distance migration of SBPHs has been reported from mid-Eastern China to Korea and Japan by westerlies in the end of May and early June (Otuka et al. Citation2012) and they can overwinter in each country, causing accumulation of different genotypes on these countries. Till now, two Chinese (Song and Liang Citation2009; Zhang et al. Citation2013) and one mid-Western Korean (Park et al., Citationunder review) SBPH mitogenomes are available, requiring additional mitogenomes for understanding genetic diversity based on geographical distribution.
SBPHs collected in Milyang, Southern part of Korea (35°29′26″N, 128°44′27″E), were selected and used for next generation sequencing by extracting DNA using CTAB-based DNA extraction method (iNtRON biotechnology, Inc., Korea). Voucher was deposited in InfoBoss Cyber Herbarium (IN; Seo B-Y, INH-00019; Korea). Raw sequences obtained from Illumina NextSeq500 (Macrogen Inc., Korea) were filtered using Trimmomatic 0.33 (Bolger et al. Citation2014), de novo assembled by Velvet 1.2.10 (Zerbino and Birney Citation2008) and MITOBim 1.9.1 (Hahn et al. Citation2013), and gaps including 634-bp-AT-rich region were closed with SOAPGapCloser 1.12 (Zhao et al. Citation2011), BWA 0.7.17, SAMtools 1.9 (Li et al. Citation2009; Li Citation2013), and PCR. Geneious R11 11.1.5 (Biomatters Ltd, Auckland, New Zealand) was used to annotate mitogenome with that of Korean SBPH (D5; MK764270; Park et al. Citationunder review).
SBPH mitogenome (D7; GenBank accession is MK862265) is 16,359 bp long, which is same as D5 (MK764270). Its nucleotide composition is AT-biased (A + T is 77.3%). It contains 13 protein-coding genes, two rRNAs, and 22 tRNAs. The control region, presumably corresponding to the single largest non-coding AT-rich region, is 1,972 bp long (A + T is 83.7%). There is only one single nucleotide polymorphism (SNP) between Korean D5 and D7, showing low-level variation. On the other hand, total 141 and 41 SNPs and 166 and 118 insertions and deletions (INDELs) were identified in comparison between D7 and two Chinese mitogenomes (NC_013706 and JX880068), respectively. A similar level of geographical variations between insect mitogenomes of the two countries has been identified in Nilaparvata lugens with 90 SNPs and 10 INDELs (Choi et al. Citation2019) and 112 SNPs and 59 INDELs (Park, Kwon, et al. Citation2019) and in Chilo supressalis with 79 SNPs and 291 INDELs (Park, Xi, et al. Citation2019).
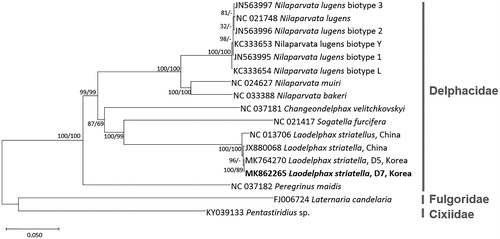
We inferred phylogenetic relationship of 17 mitgenomes covering 15 in Delphacidae and two in Cixiidae and Fulgoridae as outgroup. Seventeen mitogenomes were aligned using MAFFT 7.388 (Katoh and Standley Citation2013). Bootstrapped neighbour-joining and maximum-likelihood phylogenetic trees were constructed using MEGA X (Kumar et al. Citation2018). D7 was clustered into one clade of L. striatellus mitogenomes in monophyletic Delphacidae with high bootstrap values but has lower genetic distance with D5 than with two Chinese mitogenomes (). Additional SBPH mitogenomes in China and Korea will throw light on understanding geographical diversification of L. striatellus and tracing its origin after migration.
Figure 1. Neighbour-joining (10,000 bootstrap repeats) and maximum-likelihood (1,000 bootstrap repeats) phylogenetic trees of seven insect species in Delphacidae: four L. striatellus (MK862265 in this study, MK764270, NC_013706, and JX880068), six Nilaparvata lugens (NC_021748, JN563997, JN563996, JN563995, KC333653, and KC333654), Nilaparvata bakeri (NC_033388), Nilaparvata muiri (NC_024627), Sogatella furicefera (NC_021417), Changeondelphax velitchkovskyi (NC_037181), and Peregrinus maidis (NC_037182). As outgroup species, Pentastiridius sp. (KY039133) in Cixiidae and Laternaria candelaria (FJ006724) in Fulgoridae were used. Phylogenetic tree was drawn based on neighbour-joining tree. The numbers above branches indicate bootstrap support values of neighbour-joining and maximum-likelihood phylogenetic trees, respectively.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30:2114–2120.
- Choi NJ, Lee B-C, Park J, Park J. 2019. The complete mitochondrial genome of Nilaparvata lugens (Stål, 1854) captured in China (Hemiptera: Delphacidae): investigation of intraspecies variations between countries. Mitochondrial DNA B. 4:1677–1678.
- Hahn C, Bachmann L, Chevreux B. 2013. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads—a baiting and iterative mapping approach. Nucleic Acids Res. 41:e129–e129.
- Hibino H. 1996. Biology and epidemiology of rice viruses. Ann Rev Phytopathol. 34:249–274.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35:1547–1549.
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics. 25:2078–2079.
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv preprint arXiv:13033997.
- Otuka A, Zhou Y, Lee G-S, Matsumura M, Zhu Y, Park H-H, Liu Z, Sanada-Morimura S. 2012. Prediction of overseas migration of the small brown planthopper, Laodelphax striatellus (Hemiptera: Delphacidae) in East Asia. Appl Entomol Zool. 47:379–388.
- Park J, Jung JK, Koh YH, Park J, Seo BY. under review. The complete mitochondrial genome of Laodelphax striatellus (Fallén, 1826) (Hemiptera: Delphacidae) collected in a mid-western part of Korean peninsula. doi:10.1080/23802359.2019.1623112
- Park J, Kwon W, Park J, Kim H-J, Lee B-C, Kim Y, Choi NJ. 2019. The complete mitochondrial genome of Nilaparvata lugens (stål, 1854) captured in Korea (Hemiptera: Delphacidae). Mitochondrial DNA B. 4:1674–1676.
- Park J, Xi H, Kwon W, Park C-G, Lee W. 2019. The complete mitochondrial genome sequence of Korean Chilo suppressalis (Walker, 1863) (Lepidoptera: Crambidae). Mitochondrial DNA B. 4:850–851.
- Seo B, Kwon Y, Jung J, Kim G. 2016. Feeding behavior of the small brown planthopper, Laodelphax striatellus (Hemiptera: Delphacidae) on rice plants based on EPG waveform, honeydew excretion, and microsection analysis. Korean J Appl Entomol. 55:351–358.
- Song N, Liang A-P. 2009. Complete mitochondrial genome of the small brown planthopper, Laodelphax striatellus (Delphacidae: Hemiptera), with a novel gene order. Zool Sci. 26:851–860.
- Wilson MR, Claridge MF. 1991. Handbook for the identification of leafhoppers and planthoppers of rice. Wallingford: CAB International.
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829.
- Zhang K-J, Zhu W-C, Rong X, Zhang Y-K, Ding X-L, Liu J, Chen D-S, Du Y, Hong X-Y. 2013. The complete mitochondrial genomes of two rice planthoppers, Nilaparvata lugens and Laodelphax striatellus: conserved genome rearrangement in Delphacidae and discovery of new characteristics of atp8 and tRNA genes. BMC Genomics. 14:417.
- Zhao Q-Y, Wang Y, Kong Y-M, Luo D, Li X, Hao P. 2011. Optimizing de novo transcriptome assembly from short-read RNA-Seq data: a comparative study. BMC Bioinformatics. 12:S2.
