Abstract
Polypodiodes amoena is an important medical fern of Polypodiaceae. Its complete chloroplast genome is obtained through Illumina sequencing, which is 152,067 bp in length with a large single copy (LSC) region (81,187 bp), a small single copy (SSC) region (21,590 bp), and two inverted repeats (IRa and IRb) regions (24,645 bp). The genome encodes 130 genes, including 88 protein-coding genes, 33 tRNA genes, eight rRNA genes, and one pseudogene. ML phylogenetic analysis reveals that P. amoena is clustered with polypodiaceous ferns.
Polypodiodes amoena (Wallich ex Mettenius) Ching is an important medical fern of Polypodiaceae known as ‘Gusuibu’ for relieving rigidity of muscles and activating collaterals, and clearing heat and detoxication (National Pharmacopoeia Committee Citation2010). As a medicinal part, its rhizome is 5–7 mm in diameter, densely covered with dark-brown scales. The plant is epiphytic growing on rocks or on tree trunks with a wide distribution in south China such as Guangdong, Guangxi, and Guizhou, etc. (Zhang et al. Citation2013). In addition, Polypodiodes is a core group for investigating the controversial phylogenetic relationship among Polypodiastrum, Schellolepis, and Goniophlebium (Schneider et al. Citation2004; Lu and Li Citation2006). Therefore, the sequencing of the whole chloroplast genome of P. amoena lays a solid foundation for further exploring phylogeny of the family Polypodiaceae.
The fern was provided by South China Botanical Garden, Chinese Academy of Sciences (23°11′3.56″N, 113°21′43.28″E). The specimen is held by Herbarium of Sun Yat-sen University (SYS; voucher: SS Liu 20161021). The total genomic DNA was isolated from fresh leaves using Tiangen Plant Genomic DNA kit (Tiangen Biotech Co., Beijing, China). We constructed a paired-end genome library with an insert size of 300 bp, which was further sequenced on Illumina Hiseq 2500 platform (Illumina Inc., San Diego, CA). Approximately 2.24 Gb of sequence data were generated. After trimming and filtering through Trimmomatic v0.32 (Bolger et al. Citation2014), 2.03G clean data were de novo assembled into the chloroplast genome by Velvet v1.2.07 (Zerbino and Birney Citation2008). Annotation was conducted by tRNAscan-SE (Schattner et al. Citation2005) and DOGMA (Wyman et al. Citation2004) with default settings to identify protein-coding genes, rRNAs, and tRNAs.
The circular genome is 152,067 bp in size (Genbank accession number: MN017598) and comprises a large single copy (LSC) region (81,187 bp), a small single copy (SSC) region (21,590 bp), and two inverted repeats (IRa and IRb) regions (24,645 bp). The total GC content is 41.33%, while the corresponding values are 44.89%, 37.58%, and 40.15% in IRs, SSC, and LSC, respectively. The genome encodes 130 genes, including 88 protein-coding genes, 33 tRNA genes, eight rRNA genes, and one pseudogene. Among them, 13 genes are duplicated, including four protein-coding genes (rps12, rps7, psbA, and ycf2), five tRNA genes (trnH-GUG, trnI-GAU, trnA-UGC, trnT-UGU, and trnR-ACG) and four rRNA genes (rrn4.5, rrn5, rrn16, and rrn23). Moreover, 18 genes contain introns with 15 having one intron (ndhB, rps16, atpF, rpoC1, petB, petD, ndhA, rpl16, rpl2, trnG-UCC, trnV-UAC, trnA-UGC, trnI-GAU, trnL-UAA, and trnT-UGU), while three have three introns (ycf3, clpP, and rps12).
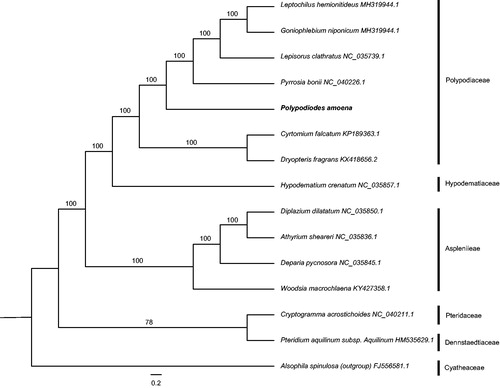
In order to assess the phylogenetic relationship among P. amoena and the other ferns, we selected 13 related complete chloroplast genomes with Alsophila spinulosa as outgroup. Their alignment was created by MAFFT (Katoh and Standley Citation2013) and further used to constructed Maximum Likelihood (ML) phylogenetic tree through RAxML with 1000 replicates (Stamatakis Citation2014). The result reveals that P. amoena is clustered with polypodiaceous fern with high bootstrap values (). This study provides new genomic resources to resolve taxonomic disputes of Polypodiaceae.
Disclosure statement
The authors declare no conflict of interests. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30:2114–2120.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Lu SG, Li CX. 2006. Phylogenetic position of the monotypic genus Metapolypodium Ching endemic to Asia: evidence from chloroplast DNA sequences of rbcL gene and rps4-trnS region. Acta Phytotaxon Sin. 44:494–502.
- National Pharmacopoeia Committee. 2010. Pharmacopoeia of People’s Republic of China. Beijing (China): Chemical Industry Press.
- Schattner P, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33:W686–W689.
- Schneider H, Smith AR, Cranfill R, Hildebrand TJ, Haufler CH, Ranker TA. 2004. Unraveling the phylogeny of polygrammoid ferns (Polypodiaceae and Grammitidaceae): exploring aspects of the diversification of epiphytic plants. Mol Phylogenet Evol. 31:1041–1063.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20:3252–3255.
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829.
- Zhang XC, Lu SG, Lin YX, Qi XP, Moore S, Xing FW, Wang FG, Hovenkamp PH, Gilbert MG, Nooteboom HP, et al. 2013. Polypodiaceae. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China. Vol. 2–3 (Pteridophytes). Beijing (China): Science Press; p. 758–850.