Abstract
Here, we report the first complete chloroplast genome of Platanthera chlorantha (Orchidaceae: Orchidoideae). The circular genome with the length of 154,260 bp possesses the typical structure consisting of a large single copy region (LSC) of 83,279 bp and a small single copy region (SSC) of 17,759 bp, separated from each other by two copies of inverted repeats (IRs) of 26,611 bp. The plastome encodes 134 genes, of which 88 were protein-coding, eight encoded ribosomal RNA, and 38 transfer RNAs. The overall GC content was 36.74%. The plastome sequence provided here constitutes a valuable resource for analyzing genetic diversity of the Orchidaceae family.
Due to their extensive variability and biotic interactions, orchids present a compelling subject for evolutionary studies (Selosse Citation2014). Chloroplast genomes provide researchers with data invaluable for studying genetic history and phylogeny and thus were already used to resolve major phylogenetic relationships between orchid subfamilies (Givnish et al. Citation2015). In this study, we sequenced for the first time the plastome of Platanthera chlorantha (Custer) Rchb. This species inhabits mostly woods, meadows, and damp heaths and is widely spread through Europe, excluding eastern, northern, and south-western edges (Hultén and Fries Citation1986). It belongs to the Orchidoideae subfamily represented by almost 3800 species. With the main focus of researchers being put on the Epidendroideae tribes (142 of 175 orchid species with complete plastomes; NCBI GenBank, as of April 2019), currently only 14 complete chloroplast genomes are available within Orchidoideae tribes (Yu et al. Citation2015; Zhu et al. Citation2016; Roma et al. Citation2018; Oh et al. Citation2019) and only one of those belongs to the member of the same genus Platanthera, namely P. japonica (Dong et al. Citation2018).
Fresh plant leaves were collected from Kalina Lisiniec, Poland (50°21′43.7″N 20°09′37.4″E) and dried in silica-gel (voucher SG-13236, Herbarium of University of Gdansk, UGDA). Extraction of the total genomic DNA was performed using DNeasy Plant Mini Kit (Qiagen), followed by sequencing library generation with Accel-NGS® 1S Plus DNA Library Kit (Swift Biosciences Inc., USA) and paired-end sequencing on Illumina HiSeq 4000. Geneious (version 10.2.4, https://www.geneious.com), with the Geneious assembling algorithm (medium-low sensitivity parameters) and a subset of 25% of the reads was used for plastome assembly. Annotations were obtained with Geneious annotations transfer procedure from P. japonica (NC_037440.1) with a 77% similarity threshold, additionally aided using GeSeq (Tillich et al. Citation2017) and followed by manual verification. Inferring of phylogenetic position of P. chlorantha was based on ML analysis performed with RAxML-NG (Kozlov et al. Citation2019) and multiple sequence alignment of full chloroplast sequences conducted with MAFFT (Katoh and Standley Citation2013).
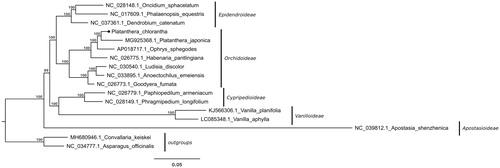
The complete circular chloroplast genome of P. chlorantha (GenBank MK937914) is a 154,260 bp long sequence, with 36.74% GC content and a typical structure consisting of two inverted repeats (IRa and IRb) of 26,611 bp each, separated by a large single-copy region (LSC) of 83,279 bp and a small single-copy region (SSC) of 17,759 bp in length. The plastome contains 134 (113 unique) genes, of which eight are rRNA genes, 38 encode tRNAs, and 88 are protein-coding. One of the ycf1 genes located at the IRb/SSC boundary is truncated but with complete open reading frame and one copy of the rpl22 gene is a pseudogene. Introns were annotated for 12 protein-coding genes and six tRNA genes. Phylogenetic analyses () showed that P. chlorantha grouped together with P. japonica and other representatives of Orchidoideae. Despite floral similarities, it is phylogenetically distant from the genus Habenaria as already noted before (Bateman et al. Citation2003). In the genus Platanthera, some species display a trend to recover biomass from the symbiotic fungi colonizing their roots (i.e. a partial heterotrophy/mixotrophy, based on their mycorrhizal fungi; see Selosse and Roy Citation2009; Yagame et al. Citation2012; references therein). Our result show plastids with apparently intact photosynthetic abilities in P. chlorantha, which is in agreement with indirect evidence for autotrophy published by Bidartondo et al. (Citation2004) for this species. The plastome sequence we provided here constitutes a valuable aid for analyzing the genetic diversity of the Orchidaceae family.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bateman RM, Hollingsworth PM, Preston J, Yi-Bo L, Pridgeon AM, Chase MW. 2003. Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae). Bot J Linn Soc. 142:1–40.
- Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ. 2004. Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc R Soc London Ser B Biol Sci. 271:1799–1806.
- Dong W-L, Wang R-N, Zhang N-Y, Fan W-B, Fang M-F, Li Z-H. 2018. Molecular evolution of chloroplast genomes of orchid species: insights into phylogenetic relationship and adaptive evolution. IJMS. 19:716.
- Givnish TJ, Spalink D, Ames M, Lyon SP, Hunter SJ, Zuluaga A, Iles WJD, Clements MA, Arroyo MTK, Leebens-Mack J, et al. 2015. Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proc R Soc B Biol Sci. 282:20151553.
- Hultén E, Fries M. 1986. Atlas of North European vascular plants: north of the Tropic of Cancer. Königstein (Federal Republic of Germany): Koeltz Scientific Books.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. 2019. RAxML-NG: a fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. https://academic.oup.com/bioinformatics/advance-article/doi/10.1093/bioinformatics/btz305/5487384
- Oh S-H, Suh HJ, Park J, Kim Y, Kim S. 2019. The complete chloroplast genome sequence of a morphotype of Goodyera schlechtendaliana (Orchidaceae) with the column appendages. Mitochondrial DNA B. 4:626–627.
- Roma L, Cozzolino S, Schlüter PM, Scopece G, Cafasso D. 2018. The complete plastid genomes of Ophrys iricolor and O. sphegodes (Orchidaceae) and comparative analyses with other orchids. PLoS One. 13:e0204174.
- Selosse M-A. 2014. The latest news from biological interactions in orchids: in love, head to toe. New Phytol. 202:337–340.
- Selosse M-A, Roy M. 2009. Green plants that feed on fungi: facts and questions about mixotrophy. Trends Plant Sci. 14:64–70.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45:W6–W11.
- Yagame T, Orihara T, Selosse M-A, Yamato M, Iwase K. 2012. Mixotrophy of Platanthera minor, an orchid associated with ectomycorrhiza-forming Ceratobasidiaceae fungi. New Phytol. 193:178–187.
- Yu C, Lian Q, Wu K, Yu S, Xie L, Wu Z. 2015. The complete chloroplast genome sequence of Anoectochilus roxburghii. Mitochondrial DNA. 27:1–2.
- Zhu S, Niu Z, Yan W, Xue Q, Ding X. 2016. The complete chloroplast genome sequence of Anoectochilus emeiensis. Mitochondrial DNA A. 27:3565–3566.