Abstract
Magnolia delavayi, a threatened plant endemic to Southwest China, is of great importance for landscaping because of its lotus-like creamy flowers. In this study, the complete chloroplast (cp) genome of M. delavayi was assembled based on the Illumina sequences. The cp genome of M. delavayi was 159,470 bp in length and contained a pair of inverted regions (IR, 26,409 bp) which were separated by the small single copy (SSC, 18,760 bp) and the large single copy (LSC, 87,892 bp) regions. It encoded 132 genes including 86 protein-coding genes, 37 tRNA genes, and eight rRNA ribosomal genes. The overall AT content of M. delavayi cp genome is 60.7%. The maximum likelihood phylogenetic analysis revealed that the species of M. delavayi was isolated first among the genus Magnolia. This result will be helpful for the conservation and phylogeny programs of the genus Magnolia.
Magnolia delavayi Franch., an evergreen tree in family Magnoliaceae, is endemic to Southwest China (Li et al. Citation2017). The species is of great importance for landscaping as well as medicine (Cao et al. Citation2004). It has been cultivated in Buddhist temples in Southwest China for hundred years because its attractive lotus-like creamy flowers () are regarded as the flowers of ‘Udumbara’ in Buddhist culture (Lin et al. Citation2003). In recent decades, more and more plants of M. delavayi has been used for local urban greening (Lin et al. Citation2003). Although it ban be propagated by grafting and tissue culture (Tang et al. Citation2014), the wild resources still go through excessive anthropogenic destruction by transplanting. Furthermore, the M. delavayi has extremely low seed-setting rate due to the special characteristics of blooming and pollination (Gong and Wu Citation1998; Li et al. Citation2017). At present, the species of M. delavayi has been seriously declined, and has been classified as “Least Concern” in the IUCN Red List of Threatened Species (Rivers and Wheeler Citation2014). Therefore, the M. delavayi should be protected effectively to avoid endangered even extinction. Previous studies of M. delavayi mainly focused on its basic biology, such as ovule number (Gong et al. Citation1999), and pollen germination (Li et al. Citation2017), but no complete chloroplast (cp) genome of M. delavayi has been reported. Here, we assembled the cp genome of M. delavayi as its basic conservation genetic resources.
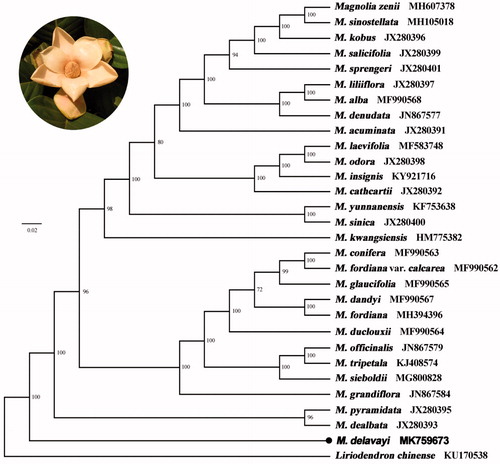
Figure 1. Maximum-likelihood phylogenetic tree for Magnolia delavayi based on 30 complete chloroplast genomes. The number on each node indicates bootstrap support value.
The total DNA from leaf tissue samples of a single individual (Location: N25°23′52″, E102°40′18″. Specimen voucher: Xu2018099, stored in the Herbarium of Kunming University of Science and Technology) was extracted with a modified CTAB method (Doyle and Doyle Citation1987). Illumina libraries were constructed, and high-throughput sequencing was carried out on the Illumina HiSeq X Ten sequencing system following the manufacturer’s protocol (Illumina, CA, USA). Approximately 2.0 Gb of clean reads data were generated after trimming with Trimmomatic v0.36 (http://www.sadellab.org/cms/index.php?page=trimmomatic) (Bolger et al. Citation2014). A combination of de novo assembly and reference-assisted mapping was applied to assemble the cp genome using Geneious R10 software (Biomatters Ltd., Auckland, New Zealand). Finally, the annotated cp genome sequence was submitted to GenBank (accession number MK759673).
The cp genome of M. delavayi was 159,470 bp in length and contained a pair of IR regions (26,409 bp) which were separated by a SSC region (18,760 bp) and a LSC region (87,892 bp). Whole cp genome encoded 132 genes including 86 protein-coding genes, 37 tRNA genes, eight rRNA ribosomal genes. In these genes, eight genes (ndhA, petB, rpoC1, rps16, trnC-ACA, trnG-UCC, trnK-UUU, and trnL-UAA) harbored one intron and six genes (clpP, ndhB, rpl2, trnA-UGC, trnG-UUC, and ycf3) had two introns. Most of genes occurred in a single copy, while six PCGs (ndhB, rpl2, rpl23, rps7, ycf2, and ycf15), seven tRNA genes (trnA-UGC, trnI-CAU, trnI-GAU, trnL-CAA, trnN-GUU, trnR-ACG, and trnV-GAC), and four rRNA genes (rrn4.5, rrn5, rrn16, and rrn23) in IR regions were duplicated. The overall AT content of M. delavayi cp genome was 60.7% and the corresponding values in LSC, SSC, and IR regions were 62.0, 65.6, and 56.8%, respectively.
A total of 29 cp genomes of Magnoliaceae together with the obtained cp genome in this study were utilized to clarify the phylogenetic position of M. delavayi, by the outgroup of Liriodendron chinense. All of the cp genome sequences were aligned using MAFFT (Katoh and Standley Citation2013) by the software Geneious R10. A maximum likelihood analysis was performed with the RAxML software using 1000 bootstrap replicates. The phylogenetic tree revealed that the species of M. delavayi was isolated first among the genus Magnolia with the support rate of 100% (). This phylogenetic result enhanced the study by Shen et al. (Citation2018) using complete cp genomes but was not consistent with the analysis by Kim et al. (Citation2001) using cp ndhF and Nie et al. (Citation2008) by nuclear genes.
The first report of complete cp genome in M. delavayi will be a valuable resource for the future studies in conservation genetics, phylogeny, and breeding in Magnolia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120.
- Cao JX, Lai GF, Wang YF, Huang YF, Luo SD. 2004. A new sesquiterpenoid from Magnolia delavayi. Chinese Chem Lett. 15:791–793.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Gong X, Lu YX, Zhang YP, Wu QA, Yue ZS. 1999. Discovery of 3-7 ovules in one carpel of Magnolia delavayi. Acta Bot Yunnan. 21:173–176.
- Gong X, Wu Q. 1998. Pollination biology of cultivated Magnolia delavayi. Acta Bot Yunnan. 20:89–93.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Kim S, Park CW, Kim YD, Suh Y. 2001. Phylogenetic relationships in family Magnoliaceae inferred from ndhF sequences. Am J Bot. 88(4):717–728.
- Li F, Xu H, Gong X, Wang S. 2017. Characteristics of pollen germination and pollen tube growth of Magnolia delavayi. Guihaia. 37:478–484.
- Lin P, Wang YC, Wang X. 2003. Application of Yunnan native tree species in Kunming urban greening. J Southwest for Univ. 23:38–42.
- Nie ZL, Wen J, Azuma H, Qiu Y, Sun H, Meng Y, Sun W, Zimmer EA. 2008. Phylogenetic and biogeographic complexity of Magnoliaceae in the Northern Hemisphere inferred from three nuclear data sets. Mol Phylogenet Evol. 48(3):1027–1040.
- Rivers MC, Wheeler L. 2014. Magnolia delavayi. The IUCN Red List of Threatened Species. 2014e.T39011A2885519. [accessed on 2019 May 01]. http://dx.doi.org/10.2305/IUCN.UK.2014-3.RLTS.T39011A2885519.en.
- Shen Y, Chen K, Gu C, Zheng S, Li M. 2018. Comparative and phylogenetic analyses of 26 Magnoliaceae species based on complete chloroplast genome sequences. Can J Forest Res. 48:1456–1469.
- Tang J, Gao Z, Liu T, Zhao X, Li W. 2014. Selection and disinfection methods of explants of Magnolia delavayi in tissue culture. Guizhou Agr Sci. 11:42–45.
