Abstract
The mitochondrial genome of Sinularia maxima was completed using next-generation sequencing (NGS) method. The mitochondrial genome is a circular molecule of 18,730 bp in length. The gene arrangements include 14 protein-coding genes (PCGs), 2 ribosomal RNA genes, and 1 tRNA (tRNA-Met). The base composition is 30.18% A, 16.47% C, 19.35% G, and 33.99% T, with an A + T content of 64.18%. With regard to the phylogenetic analysis, members of genus Sinularia were clustered in different clades.
The genus Sinularia May, 1898 is one of the most widespread Octocorallia soft corals has been distributed in a wide range of habitats (Fabricius and Alderslade Citation2001). To date, the complete mitogenome of three species of Sinularia including Sinularia ceramensis (MK292119), Sinularia cf. cruciate (NC_034318) and Sinularia peculiaris (NC_018379) have been sequenced. In the present study, the complete mitochondrial genome of Sinularia maxima Verseveldt, 1971 (GenBank: MN485891) was analyzed using next-generation sequencing.
A specimen of S. maxima was collected from the South China Sea (West Island, Sanya, Hainan province, China; 18°14′ 8.75″N, 109° 22′39.10″ E) and stored in Hainan Tropical Ocean University Museum of Zoology (NO.0001-Sm). Taxonomical status of the specimen was identified by PuCAs-mtMutS (Benayahu et al. Citation2018) and PuCAs-28S (Quattrini et al. Citation2019). The whole DNA was extracted using Rapid Animal Genomic DNA Isolation Kit (Sangon Biotech Co., Ltd., Shanghai, CN; NO. B518221). A genomic library was made by paired-end (2 × 150 bp) next-generation sequencing, using the Illumina HiSeq X-ten sequencing platform (Asem et al. Citation2019). FastQC programme was utilized to check quality of sequencing reads (Andrews Citation2010) and the sequences were annotated and assembled to the Sinularia mitochondrial genome (Sinularia ceramensis, MK292119) with Spades v3.9.0 (Bankevich et al. Citation2012) and bowtie v2.2.9 (Langmead and Salzberg Citation2012). Putative tRNA gene was established using ARWEN (http://130.235.46.10/ARWEN/) online software. All genes were annotated based on gene order on the reference mitochondrial map using BLAST analysis (https://blast.ncbi.nlm.nih.gov). Additionally, to annotate PCGs and the position of start and stop codons were re-considered.
The complete mitogenome of S. maxima was 18,730 bp in length, with 14 protein-coding genes (PCGs), two ribosomal RNAs (rRNAs) and one transfer RNA (tRNA-Met). We found that tRNA-Met can be folded into typical clover-leaf secondary structures with 46.6% of GC content. The overall nucleotide composition of the major strand of the S. maxima mitogenome was as follows: 30.18% A, 16.47% C, 19.35% G, and 33.99% T, with a total A + T content of 64.18%.
The tRNA-Met and four protein-coding genes (COX3, ATP6, ATP8, and COX2) were located on the L strand. All PCGs began with common ATG start codon. Stop codons included eight TAG (ND1, CYTB, ND6, ND3, ND2, ND5, COX3, and COX2), five TAA (ND4L, mutS, ND4, ATP6, and ATP8) and a non-complete codons T- (COX1).
The 12S ribosomal RNA and 16S ribosomal RNA were encoded on H strand from 1583 to 2633 (1051 bp) and 9147 to 11115 (1969 bp), respectively, with 12S having a rather higher G + C content (43.58% vs. 41.24%). A single overlap and the longest gap were found between and ND2/ND5 (−13 bp) and COX2/COX1 (112 bp), respectively.
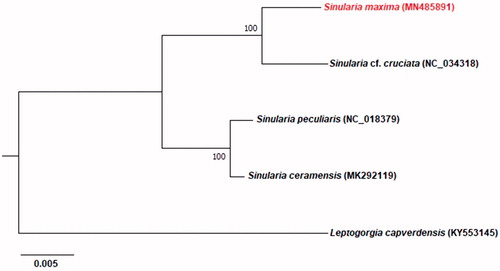
A phylogenetic analysis of four completed Sinularia mitogenomes was established based on and an outgroup (Leptogorgia capverdensis, KY553145). The concatenated dataset for nucleotides contained 14 PCGs and two ribosomal RNAs. The maximum-likelihood (ML) phylogenetic analysis was performed using the software MEGA X (Kumar et al. Citation2018). Regarding phylogenetic tree, Sinularia is divided into two clads including Sinularia cf. cruciate + Sinularia maxima and Sinularia peculiaris + Sinularia ceramensis ().
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the manuscript.
Additional information
Funding
References
- Andrews S. 2010. FastQC: A quality control tool for high throughput sequence data [accessed 2018 Oct 4]. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Asem A, Lu H, Wang P, Li W. 2019. The complete mitochondrial genome of Sinularia ceramensis Verseveldt, 1977 (octocorallia: Alcyonacea) using next-generation sequencing. Mitochondr DNA B. 4:815–816.
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19:455–477.
- Benayahu Y, van Ofwegen LP, Dai C-F, Jeng MS, Soong K, Shlagman A, Du SW, Hong P, Imam NH, Chung A, et al. 2018. The octocorals of Dongsha Atoll (South China Sea): an iterative approach to species identification using classical taxonomy and molecular barcodes. Zool Stud. 57:50.
- Fabricius K, Alderslade P. 2001. Soft corals and sea fans: a comprehensive guide to the tropical shallow-water genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Townsville: Australian Institute of Marine Science.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35:1547–1549.
- Langmead B, Salzberg S. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 9:357–359.
- Quattrini AM, Wu T, Soong K, Jeng M-S, Benayahu Y, McFadden CS. 2019. A next generation approach to species delimitation reveals the role of hybridization in a cryptic species complex of corals. BMC Evol Biol. 19:116.