Abstract
Amphiura is a widely distributed genus of Ophiuroidea from various environment, play an important role in evolution. Here, we reported a complete mitochondrial genome of Amphiura sp. which was collected from a deep sea seamount in the West Pacific. The mitogenome is 15,457 in length, including 13 protein-coding genes, 2 rRNA genes, and 22 tRNA genes. All genes are arranged in the same order of published mitogenomes in the same genus. The phylogenetic analysis support monophyly of the family Amphiuridae but not for the genus Amphiura.
Amphiura is one of the largest genera of Ophiuroidea reported from various environments (Stöhr and Segonzac Citation2005; Cecchetto et al. Citation2017; Alitto et al. Citation2018). Many species from this genus have been studied well about their biology, physiology and life history (Czarkwiani et al. Citation2016; Delroisse et al. Citation2017). However, very few studies have been published about Amphiura from deep sea (Hendler and Tran Citation2001). Recently, studies focussed on mitochondrial genome revealed that Ophiuroidea has undergone more complex gene rearrangements than other classes of echinoderms (Galaska et al. Citation2019; Lee et al. Citation2019). In this paper, the complete mitochondrial genome from a deep-sea Amphiura sp. was characterized in order to provide genetic information for further phylogenetic analysis.
The specimen was collected from the Weijia Guyot (156°28'E, 12°37'N, 1995 m depth) using ROV HAIMA, and was identified as Amphiura sp. based on the morphological characters. The specimen (RSIO41201) and its DNA (DNASIO41201) are deposited in the Repository of the Second Institute of Oceanography. Genomic DNA was extracted with a DNeasy Blood & Tissue Kit (QIAGEN, Valencia, CA), COI and 16S were first amplified and sequenced with specific primers (Palumbi et al. Citation1991; O'Hara et al. Citation2014), then the two partial mitochondrial genome sequences (COI-16S and 16S-COI) were amplified by long PCR specific primers designed according to COI and 16S sequences, then sequenced by NGS on Illumina HiSeq XTen platform (Illumina Inc. San Diego, CA). About 1G clean data of each partial sequences were de novo assembled using SPAdes-3.12 (Nurk et al. Citation2013). The remaining gaps were amplified with the species-specific primers designed according to the obtained sequences. A contig with a length of 15,457 bp was considered as the mitogenome (GenBank accession number: MN296491), on which protein-coding genes (PCGs), ribosomal RNA (rRNA) genes, and transfer RNA (tRNA) genes were annotated with MITOS2 WebServer (Donath et al. Citationin press). The boundaries of PCGs were manually adjusted by comparison with published Ophiuroid mitogenomes. Alignments of the 13 PCGs were carried out by Geneious Prime with default settings. Phylogenetic relationships were estimated using maximum likelihood, which performed by raxmlGUI 1.5b2 (Silvestro and Michalak Citation2012) using a selection of ML + thorough bootstrap with 1000 replicates and the GTR + I + G model of substitution for the nucleotide dataset. FigTree v1.4.3 (Rambaut Citation2016) was used to visualize tree files.
The complete mitogenome of Amphiura sp. contains 13 PCGs, two rRNA genes, and 22 tRNA genes. All PCGs start with ATG, except for ND6, which use GTG as the start codon, and CYTB use ATT as the start codon; most PCGs end with TAA codon, with the exception of ND6, CYTB, ND2, and ND1, which terminate with TAG codon, and COX2 terminate with TA codon ().
Figure 1. Phylogenetic tree of maximum-likelihood (ML) method based on the concatenated amino acid (AA) sequences of 13 PCGs of Amphiura sp. (MN296491) and 18 other ophiuroids.
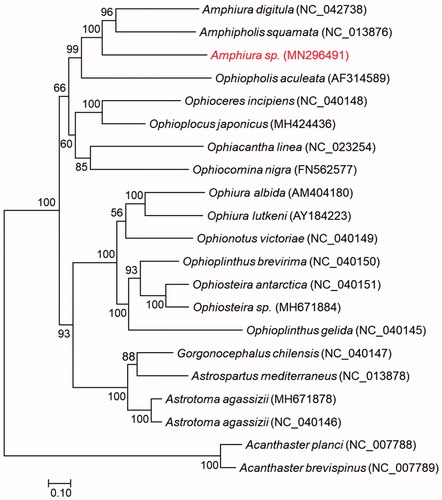
The gene orders are identical to the two published mitogenomes from the same genus, further confirming the conservative gene order among congeners (Lee et al. Citation2019). Phylogeny inference with maximum likelihood robustly supports the monophyletic Amphiuridae but not to the genus Amphiura with Amphipholis squamata located between our species and Amphiura digitula.
Acknowledgements
The authors thank the HAIMA team for their help in the specimen collection during the expedition.
Disclosure statement
The authors declare no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Alitto RAS, Bueno ML, Guilherme PDB, Domenico MD, Christensen AB, Borges M. 2018. Shalow-water brittle stars (Echinodermata: Ophiuroidea) from Araçá Bay (Southeastern Brazil), with spatial distribution considerations. Zootaxa. 4405:1–66.
- Cecchetto M, Alvaro MC, Ghiglione C, Guzzi A, Mazzoli C, Piazza P, Schiaparelli S. 2017. Distributional records of Antarctic and sub-Antarctic Ophiuroidea from samples curated at the Italian National Antarctic Museum (MNA): check-list update of the group in the Tera Nova Bay area (Ross Sea) and launch of the MNA 3D model ‘virtual gallery’. Zookeys. 705:61–79.
- Czarkwiani A, Ferrario C, Dylus DV, Sugni M, Oliveri P. 2016. Skeletal regeneration in the brittle star Amphiura filiformis. Front Zool. 13:18.
- Delroisse J, Ullrich-Lüter E, Blaue S, Eeckhaut I, Flammang P, Mallefet J. 2017. Fine structure of the luminous spines and luciferase detection in the brittle star Amphiura filiformis. Zoologischer Anzeiger. 269:1–12.
- Donath A, Juhling F, Al-Arab M, Stadler PF, Middendorf M, Bernt M. In press. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 1–10.
- Galaska MP, Li Y, Kocot KM, Mahon AR, Halanych KM. 2019. Conservation of mitochondrial genome arrangements in brittle stars (Echinodermata, Ophiuroidea). Mol Phylogenetics Evol. 130:115–120.
- Hendler G, Tran LU. 2001. Reproductive biology of a deep-sea brittle star Amphiura carchara (Echinodermata: Ophiuroidea). Mar Biol. 138:113–123.
- Lee T, Bae YJ, Shin S. 2019. Mitochondrial gene rearrangement and phylogenetic relationships in the Amphilepidida and Ophiacanthida (Echinodermata, Ophiuroidea). Mar Biol Res. 15:26–35.
- Nurk S, Bankevich A, Antipov D, Nurk S, Gurevich A, Korobeynikov A, Lapidus A, Prjibelsky A, Pyshkin A, Alexander SA, Sirotkin Y. 2013. Assembling genomes and mini-metagenomes from highly chimeric reads(C). Annual International Conference on Research in Computational Molecular Biology. Berlin, Heidelberg: Springer; pp. 158–170.
- O'Hara TD, England PR, Gunasekera RM. 2014. Limited phylogeographic structure for five bathyal ophiuroids at continental scales. Deep Sea Res Part I. 84:18–28.
- Palumbi SR, Martin A, Romano S, Mcmillan WO, Stice L, Grabowsky G. 1991. A simple fool’s guide to PCR. Honolulu, HI: University of Hawaii Press.
- Rambaut A. 2016. Figtree 1.4.3; [accessed 2019 Jan 5]. http://tree.bio.ed.ac.uk/software/figtree/.
- Silvestro D, Michalak I. 2012. raxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 12:335–337.
- Stöhr S, Segonzac M. 2005. Deep-sea ophiuroids (Echinodermata) from reducing and non-reducing environments in the North Atlantic Ocean. J Mar Biol Ass. 85:383–402.
