Abstract
Morinda citrifolia L. (Rubiaceae), commonly called noni, is a medicinal plant that is often used as botanical dietary supplement. This study is the first to report and characterize the complete chloroplast genome of M. citrifolia. We found that it contains 153,113 bp with a GC content of 38.05%, consisting of two inverted repeat regions (IRs, 25,588 bp), a large single-copy region (LSC, 83,974 bp), and a small single copy (SSC, 17,963 bp) region. One hundred and twenty-five genes were annotated, including 84 protein-coding genes, 33 transfer RNA (tRNA) genes, and 8 ribosomal RNA (rRNA) genes. Phylogenetic analysis showed that M. citrifolia and Gynochthodes officinalis were closely related. Overall, this study provided a wealth of information for a follow-up phylogenetic and evolutionary study of the Gentianales.
Morinda citrifolia L. (Rubiaceae), commonly called noni (Arunachalam Citation2018), is a medicinal plant that is also used as botanical dietary supplement (Pawlus and Kinghorn Citation2007). The genus Morinda includes approximately 80 species, mostly of Old World origin, and M. citrifolia is distributed the Pacific and also in tropical America (Morton Citation1992). Because of its suggested effects against cancer (Sharma et al. Citation2016), dyslipidemia (Mandukhail et al. Citation2010), inflammation (Mckoy et al. Citation2002), and immunostimulant properties (Brown Citation2012), many parts of the M. citrifolia tree are utilized in medicines, including the roots, leaves, fruit, and seeds (Dixon et al. Citation1999; Torres et al. Citation2017).
In recent years, M. citrifolia has garnered increasing attention because of its health-promoting properties, which has prompted an increase of research on its phytochemical constituents and biological activity (Pawlus and Kinghorn Citation2007). However, few studies have been conducted on the nuclear, mitochondrial, and chloroplast genomes of M. citrifolia. The chloroplast genome contains abundant genetic information, thus is a powerful tool for studying the evolutionary relationships of species.
Therefore, we have sequenced and analyzed the chloroplast genome of M. citrifolia. Healthy young leaves of rooted M. citrifolia plants were collected from Xishuangbanna Tropical Flowers and Plants Garden (N 22°01′6.10″ and E 100°47′18.99″). The genomic DNA was isolated from the leaves using a Dneasy Plant Mini Kit (Qiagen). The DNA was stored in an ultra-low temperature specimen library of Yunnan Institute of Tropical Crops (specimen accession number: YITC-2019-FZ-M-002) after quality control. The DNA sequence data of M. citrifolia were obtained by the Roche/454 system (Roche 454 Life Sciences) and assembled using the CLC Genomics Workbench v3.6 (http://www.clcbio.com). The chloroplast genome was annotated by DOGMA (Wyman et al. Citation2004) and manually corrected. The complete chloroplast genome sequence and annotation results of M. citrifolia were submitted to GenBank with the accession number of MN699649.
The complete, circular chloroplast genome of M. citrifolia contains 153,113 bp including 30.70% A, 31.24% T, 18.68% G, and 19.38% C, with a GC content of 38.05%. The genome is composed of two inverted repeat regions (IRs, 25,588 bp), a large single-copy region (LSC, 83,974 bp), and a small single copy (SSC, 17,963 bp) region. One hundred and twenty-five genes were annotated, including 84 protein-coding genes, 33 transfer RNA (tRNA) genes, and 8 ribosomal RNA (rRNA) genes. In terms of gene function, these protein-coding genes include photosystem I/II, cytochrome b/f complex, ATP synthase, and NADH dehydrogenase.
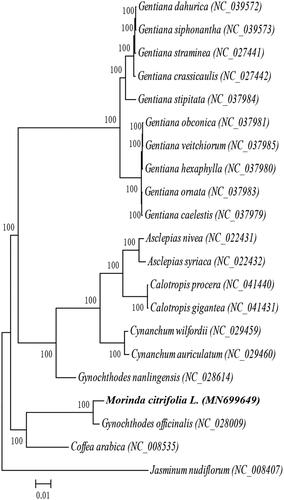
A phylogenetic analysis was conducted on the complete chloroplast genome sequences of M. citrifolia and 19 other Gentianales species, Jasminum nudiflorum (Lamiales) was used as the outgroup (). MAFFT (Katoh and Standley Citation2013) and MEGA7.0 (Kumar et al. Citation2016) were used for multiple sequence alignment and a maximum likelihood (ML) analysis. The results showed that M. citrifolia and Gynochthodes officinalis were closely related. This study provides a wealth of information for future phylogenetic and evolutionary studies on the Gentianales.
Figure 1. Maximum-likelihood phylogenetic tree of Morinda citrifolia L. and 19 other species which belong to Gentianales order based on complete chloroplast sequences, Jasminum nudiflorum (Lamiales order) was used as the outgroup. Numbers in the nodes are bootstrap values from 1000 replicates, bootstrap values are shown above the nodes. The species and chloroplast genome accession number for tree construction shown below: Gentiana dahurica (NC_039572), Gentiana siphonantha (NC_039573), Gentiana straminea (NC_027441), Gentiana crassicaulis (NC_027442), Gentiana stipitata (NC_037984), Gentiana obconica (NC_037981), Gentiana veitchiorum (NC_037985), Gentiana hexaphylla (NC_037980), Gentiana ornata (NC_037983), Gentiana caelestis (NC_037979), Asclepias nivea (NC_022431), Asclepias syriaca (NC_022432), Calotropis procera (NC_041440), Calotropis gigantea (NC_041431), Cynanchum wilfordii (NC_029459), Cynanchum auriculatum (NC_029460), Gynochthodes nanlingensis (NC_028614), Morinda citrifolia L. (MN699649), Gynochthodes officinalis (NC_028009), Coffea arabica (NC_008535), Jasminum nudiflorum (NC_008407).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arunachalam V. 2018. Morinda citrifolia L. (Rubiaceae): a multi-purpose tree for coastal ecosystems and its variability in Konkan region of India. Genet Resour Crop Evol. 65(6):1751–1765.
- Brown AC. 2012. Anticancer activity of Morinda citrifolia (noni) fruit: a review. Phytother Res. 26(10):1427–1440.
- Dixon AR, Mcmillen H, Etkin NL. 1999. Ferment this: the transformation of noni, a traditional polynesian medicine (Morinda Citrifolia, Rubiaceae). Econ Bot. 53(1):51–68.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870.
- Mandukhail SUR, Aziz N, Gilani AH. 2010. Studies on antidyslipidemic effects of Morinda citrifolia (noni) fruit, leaves and root extracts. Lipids Health Dis. 9(1):88.
- Mckoy MLG, Thomas EA, Simon OR. 2002. Preliminary investigation of the anti-inflammatory properties of an aqueous extract from Morinda citrifolia (noni). Proc West Pharmacol Soc. 45:76–78.
- Morton JF. 1992. The ocean-going noni, or Indian Mulberry (Morinda citrifolia, Rubiaceae) and some of its “colorful” relatives. Econ Bot. 46(3):241–256.
- Pawlus AD, Kinghorn DA. 2007. Review of the ethnobotany, chemistry, biological activity and safety of the botanical dietary supplement Morinda citrifolia (noni). J Pharm Pharmacol. 59(12):1587–1609.
- Sharma K, Pachauri SD, Khandelwal K, Ahmad H, Arya A, Biala P, Agrawal S, Pandey RR, Srivastava A, Srivastav A, et al. 2016. Anticancer effects of extracts from the fruit of Morinda citrifolia (noni) in breast cancer cell lines. Drug Res. 66(3):141–147.
- Torres MAO, de Fátima Braga Magalhães I, Mondêgo-Oliveira R, de Sá JC, Rocha AL, Abreu-Silva AL. 2017. One plant, many uses: a review of the pharmacological applications of Morinda citrifolia. Phytother Res. 31(7):971.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20(17):3252–3255.
