Abstract
The deep-sea blind lobster Willemoesia forceps A. Milne-Edwards, 1880 was collected at a water depth of 3433 m and is reported for the first time from the Central Indian Ridge (CIR). The species was identified based on morphological examination and supported by mitochondrial Cytochrome Oxidase Subunit I (mtCOI) gene. The phylogenetic analysis shows that W. leptodactyla is sister species of W. forceps. Our findings expand the distributional range of the specimen in the southern hemisphere.
Deep-sea blind lobsters have been classified under the Polychelidae family since all extant taxa possess reduced eyes and live in deep oceanic waters (Ahyong Citation2009). Interestingly, the deep-sea lobsters are home to the eyeless (blind), claw-footed polychelids, ‘living fossils’, the long-extinct eryonids. The extant Polychelidae family was most morphologically diverse during the Mesozoic period (Audo et al. Citation2014). The distribution of W. forceps is presently known from the West Indies, the Caribbean Sea, Sargasso Sea, West Africa, eastern Australia (Queensland), Taiwan (Ahyong Citation2012; Chang et al. Citation2013, Citation2014). The CIR is part of a ∼65,000 km long global mid-oceanic ridge network that traverses the bottom of the world’s oceans. It is a north-south-trending ridge extending from the Rodriguez Triple Junction to the equator at 25°S. Many known hydrothermal vent fields viz., Edmond (Van Dover et al. Citation2001), Kairei (Hashimoto et al. Citation2001), Dodo (Nakamura et al. Citation2012), and Solitaire (Kawagucci et al. Citation2008, Citation2016) are located in the vicinity of our study area along the CIR. The studied specimen was collected in January 2017 (Lat: 23°11′15″S; Long: 69°13′18″E, cruise no. MGS 13) during a box core operation. The specimen was identified based on morphological traits (Galil Citation2000; Chang et al. Citation2013, Citation2014). The Willemoesia forceps has the lateral margin behind the postcervical with more than 20 spines with 8–16: 5–8: 39–42. Galil (Citation2000) and Ahyong (2012) also reported a similar range of 14:13–15:27–30 and 14–19:14–15: 29–40, respectively. The specimen has been deposited at the NCPOR sample repository (voucher no.: NCPOR-CIO3433).
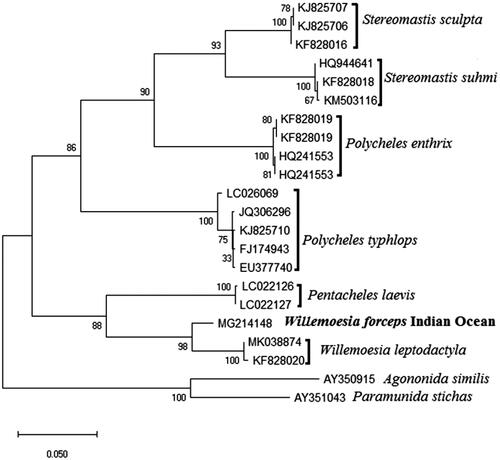
The experimental protocols and data analysis methods were followed as described in Periasamy and Ingole (Citation2019). Identified sequence has been deposited in NCBI GenBank (accession No.MG214148). The phylogenetic tree was plotted to visualize similar topologies after comparing mtCOI gene sequences of closely associated polychelids species such as Polycheles typhlops, P. enthrix, Stereomastis panglao, S. sculpta, S. suhmi, Pentacheles laevis, and Willemoesia leptodactyla were taken from NCBI website. Out-groups used were Paramunidas tichas, Agononida similis species. The phylogenetic tree of deep-sea blind lobster W. forceps species showed within Polychelidae family was divided into two groups: P. typhlops, P. enthrix, S. panglao, S. sculpta, S. suhmi consist of the first clades which are sister-group to the remaining genus such as P. laevis, Willemoesia leptodactyla, and W. forceps belong to the second group. The phylogenetics of Willemoesia-related Polychelidae and mtCOI phylogenetic reconstruction of the Willemoesia-related Polychelidae resulted in two principal group clades, each with a paralogous pair of sequence from CIR. The phylogenetic reconstruction of the mtCOI gene for Willemoesia species () showed a clear differentiation between described species and high genetic divergence between W. forceps and W. leptodactyla. The current distribution of W. forceps appears genetically correlated and suggests the species has boundless distribution and has become an independent population. Thus, considering the commercial mining potential of Indian Ocean, any information on the ecology and evolution of deep-sea blind lobster will be utmost importance for the management and conservation of deep-sea biodiversity.
Acknowledgments
The authors thank the Director of ESSO-National Center for Polar and Ocean Research (NCPOR) for the facilities. We would like to thank Professor Tin–Yam Chan, National Taiwan Ocean University, Keelung, Taiwan for confirming the taxonomic identification and providing valuable inputs in improvising the manuscript. This is NCPOR contribution No J-52/2019-20….
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahyong ST. 2009. The polychelidan lobsters: phylogeny and systematics (Polychelida: Polychelidae). In: Martin JW, Crandall KA, Felder DL, editors. Decapod crustacean phylogenetics. Boca Raton, FL: CRC Press/Taylor & Francis Group; p. 369–396.
- Ahyong ST. 2012. Polychelid lobsters (Decapoda: Polychelida: Polychelidae) collected by the CIDARIS Expeditions off Central Queensland, with a summary of Australian and New Zealand distributions. Memoirs of the Queensland Museum. 56:1–7.
- Audo D, Schweigert G, Saint Martin JP, Charbonnier S. 2014. High biodiversity in Polychelida crustaceans from the Jurassic La Voulte-sur-Rhône Lagerstätte. Geodiversitas. 36(4):489–525.
- Chang SC, Ahyong ST, Chan TY. 2013. New records of deep-sea blind lobsters (Crustacea: Decapoda: Polychelidae) from Taiwan. J Mar Sci Tech. 21:8–4.
- Chang SC, Chan TY, Ahyong ST. 2014. Two new species of the rare lobster genus Thaumastocheles Wood-Mason, 1874 (Reptantia: Nephropidae) discovered from recent deep-sea expeditions in the Indo-West Pacific. J Crust Biol. 34(1):107–122.
- Galil BS. 2000. Crustacea Decapoda: review of the genera and species of the family Polychelidae Wood-Mason, 1874, Résultats des Campagnes MUSORSTOM, Volume 21. Mémoires du Muséum National D’Histoirenaturelle. 184:285–387.
- Hashimoto J, Ohta S, Gamo T, Chiba H, Yamaguchi T, Tsuchida S, Okudaira T, Watabe H, Yamanaka T, Kitazawa M. 2001. First hydrothermal vent communities from the Indian Ocean discovered. Zool Sci. 18(5):717–721.
- Kawagucci S, Miyazaki J, Noguchi T, Okamura K, Shibuya T, Watsuji T, et al. 2016. Fluid chemistry in the Solitaire and Dodo hydrothermal fields of the Central Indian Ridge. Geofluids. 5:988–1005.
- Kawagucci S, Okamura K, Kiyota K, Tsunogai U, Sano Y, Tamaki K. Gamo T 2008. Methane, manganese, and helium-3 in newly discovered hydrothermal plumes over the Central Indian Ridge, 18°–20°S. Geochem Geophy Geosy. 9:Q10002.
- Nakamura K, Watanabe H, Miyazaki J, Takai K, Kawagucci S, Noguchi T, Nemoto S, Watsuji T-o, Matsuzaki T, Shibuya T, et al. 2012. Discovery of new hydrothermal activity and chemosynthetic fauna on the Central Indian Ridge at 18–20 S. PLoS One. 7(3):e32965.
- Periasamy R, Ingole B. 2019. Genetic variation of reef-building polychaete Sabellaria chandraae in the south-eastern Arabian Sea based on mitochondrial COI gene sequences. Mitochondr DNA B. 4(2):3291–3292.
- Van Dover C L, Humphris S E, Fornari D, Cavanaugh C M, Collier R, Goffredi S K, Hashimoto J, Lilley M D, Reysenbach A L, Shank T M, et al. 2001. Biogeography and ecological setting of Indian Ocean hydrothermal vents. Science. 294(5543):818–823.