Abstract
We report the complete mitogenome (Mt) sequences of Aedes (Howardina) busckii and Aedes (Ochlerotatus) taeniorhynchus. The sequences were extracted from one individual per species from the Dutch Leeward Island of Saba. The length of the Ae. busckii Mt was 16,794 bp with 80.6% AT content. The Ae. taeniorhynchus Mt was 16,216 bp long with 79.8% AT content. These are the first full Mt sequences available for these species.
Keywords:
Aedes (Howardina) busckii (Coquillett, 1906) and Aedes (Ochlerotatus) taeniorhynchus (Wiedemann, 1821), also known as Howardina buskii and Ochlerotatus (Culicelsa) taeniorhynchus (Harbach Citation2019), were first documented in the Lesser Antilles in the 1950s (Van der Kuyp Citation1954). Immature Ae. busckii mosquitoes, a species restricted to the Caribbean Islands, are found in water-filled bracts of Heliconia plants or tree holes on Saba (Bass and Bass Citation2011; Boerlijst et al. Citation2019). Aedes taeniorhynchus, a mostly salt marsh and brackish water species, found in Southern, Latin and North America (south of 48°North) (Carpenter and La Casse Citation1955), is occasionally found in fresh-water ponds and wells on Saba (Van der Kuyp Citation1954; Boerlijst et al. Citation2019). Here, we report two complete mitogenome sequences of wild-caught Ae. busckii and Ae. taeniorhynchus from Saba (17°38′N 63°15′W). The specimens sequenced were both collected as adult females in BG-sentinel mosquito traps (Biogents – Regensburg, Germany) baited with just the chemical lure.
DNA extraction and library preparations were conducted using the published protocol as in Schmidt et al. (Citation2018). The libraries were sequenced for 150 bp paired-end reads using a HiSeq 4000 instrument at UC Davis. Raw-sequencing reads were trimmed using Trimmomatic v0.36 (Bolger et al. Citation2014). Mt contigs were assembled for each individual using NOVOPlasty v2.6.7 (Dierckxsens et al. Citation2017).
The length of the Aedes busckii Mt (Genbank: MN626443) was 16,542 bp and the Aedes taeniorhynchus Mt (Genbank: MN626442) was 16,216 bp. The (A + T)% was approximately 80% for both species. The COI fragment spanning 1475–2132bp of Ae. busckii sequence was 99.8% identical to the published Ae. busckii COI sequences originating from Saba (Boerlijst et al. Citation2019). The COI fragment spanning 1474–2131bp of Ae. taeniorhynchus average pairwise identity was 99.4% identical to COI sequences of Ae. taeniorhynchus deposited in GenBank of specimens from Mexico, Columbia, and other unspecified locations (Gibson et al. Citation2012; Hoyos-Lopez et al. Citation2015; Soghigian et al. Citation2017; Toro-Cantillo et al. Citation2017).
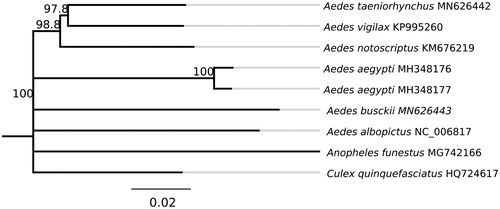
A phylogeny including other disease vector mosquito species is shown in . Jukes-Cantor model was used to calculate pairwise genetic distances and the neighbor-joining method was used to build the phylogenetic tree using Geneious Software version R9 (Kearse et al. Citation2012). Aedes taeniorhynchus was most closely related to Ae. vigilax, the other Ochlerotatus included in the phylogeny, with 93.6% sequence similarity. Ae. busckii was more distantly related to other Aedes species ranging from 86.9 to 89.9% sequence similarity. Extracted DNA samples are kept in the Vector Genetics Laboratory at UC Davis with Accession IDs 2019SABA104 (Ae. busckii) and 2018SABA301 (Ae. taeniorhynchus) and metadata records are available through https://popi.ucdavis.edu/
Figure 1. Phylogenetic relationships based on Mt sequences of related mosquito species. Genbank IDs used in this analysis are provided next to each species name. Numbers at nodes indicate bootstrap values out of 500 replicates. Anopheles gambiae was used as an outgroup. Branch length scale bar indicates relative differences (0.02 = 2% nucleotide difference).
The mitogenomes were annotated using MITOS (Bernt et al. Citation2013) under default settings and the invertebrate genetic code for mitochondria followed that used in Song and Zhang (Citation2018). The gene start and end positions and gene orders were by and large consistent with published annotations of mosquito Mt sequences used in the phylogeny.
Acknowledgements
We thank Dr. Lutz Froenicke and his team at the UC Davis DNA Technologies Core for providing sequencing services. We thank the Vector control team in Saba, Sint Maarten, and Sint Eustatius for collecting the mosquito samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bass CM, Bass D. 2011. Aquatic invertebrate community structure in water-filled bracts of Heliconia caribaea (Heliconiaceae) on Saba, West Indies. Living World. 60–65.
- Bernt M, Donath A, Juhling F, Externbrink F, Florentz C, Fritzsch G, Putz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Boerlijst SP, Trimbos KB, Van der Beek JG, Dijkstra KDB, Van der Hoorn BB, Schrama M. 2019. Field evaluation of DNA based biodiversity monitoring of Caribbean mosquitoes. Front Ecol Evol. 7:240.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120.
- Carpenter SJ, La Casse WJ. 1955. Mosquitoes of North America (north of Mexico). Berkeley: University of California Press.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18.
- Gibson CM, Kao RH, Blevins KK, Travers PD. 2012. Integrative taxonomy for continental-scale terrestrial insect observations. PLoS One. 7(5):e37528.
- Harbach R. 2019. Mosquito taxonomic inventory. [accessed 2020 Feb 14]. http://mosquito-taxonomic-inventory.info/sites/mosquito-taxonomic-inventory.info/files/Valid%20Species%20List_73.pdf.
- Hoyos-Lopez R, Uribe S, Gallego-Goez J. 2015. Utility of DNA barcode for mosquitoes potentially vectors for arboviruses in a Mangrove from Caribbean Colombian Coast. [accessed 2020 Feb 14]. https://www.ncbi.nlm.nih.gov/nuccore/KT766538.1.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649.
- Schmidt H, Hanemaaijer MJ, Cornel AJ, Lanzaro GC, Braack L, Lee Y. 2018. Complete mitogenome sequence of Aedes (Stegomyia) aegypti derived from field isolates from California and South Africa. Mitochondrial DNA B. 3(2):994–995.
- Soghigian J, Andreadis TG, Livdahl TP. 2017. From ground pools to treeholes: convergent evolution of habitat and phenotype in Aedes mosquitoes. BMC Evol Biol. 17(1):262.
- Song N, Zhang H. 2018. The mitochondrial genomes of phytophagous scarab beetles and systematic implications. J Insect Sci. 18(6:)1–11.
- Toro-Cantillo A, Gallego-Gomez J, Hoyos R. Forthcoming 2017. DNA barcode reveals potential vectors of VEEV in coastal zones from Cordoba Colombia. [accessed 2020 Feb 14]. https://www.ncbi.nlm.nih.gov/nuccore/KY859919.1.
- Van der Kuyp E. 1954. Mosquitoes of the Netherlands Antilles and their hygienic importance. Studies on the Fauna of Curaçao and Other Caribbean Islands. 5(23):37–114.
