Abstract
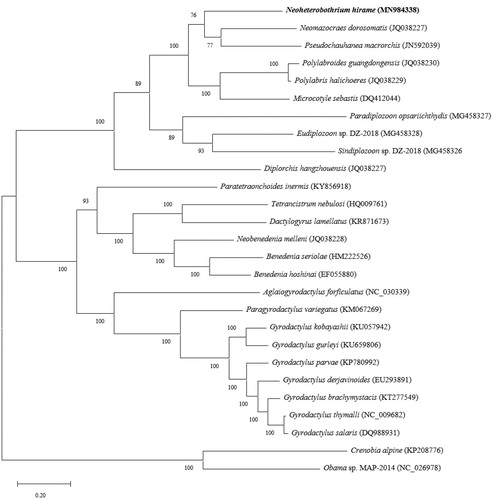
The mitogenome of Neoheterobothrium hirame from olive flounder (Paralichthys olivaceus) was obtained and analyzed in this study. The N. hirame circular mitogenome was 14,588 bp in size, consisting of 12 protein-coding genes (PCGs) lacking atp8 gene, 22 tRNA genes, 2 rRNA genes, 1 repeat region and several non-coding regions. The arrangement of PCGs was identical with those of other polyophistocotylean mitogenomes. The phylogenetic tree revealed that N. hirame clustered with Pseudochauhanea macrorchis, Neomazocraes dorsomatis, forming a sister group relationship to microcotylids.
We determined the complete mitochondrial (mt) genome of Neoheterobothrium hirame (Monogenea: Diclidophoridae). We collected N. hirame from the buccal cavity wall of olive flounder (Paralichthys olivaceus) maintained in the aquaculture facility of Chonnam National University, Yeosu, Korea (34.46 N, 127.42 E). They were identified by morphology (Ogawa Citation1999) and the ITS sequence (GenBank accession no. FJ480934.1), and DNA was sequenced by MGISEQ-2000. The slide specimen (specimen number GWFSC-181201) was deposited in the Department of Marine Bioscience, Gangneung-Wonju National University. Mitogenome annotation was conducted with MITOS (Bernt et al. Citation2013) and tRNAScan (Chan and Lowe Citation2019) and BLAST against Pseudochauhanea macrorchis mitogenome (Zhang et al. Citation2012). Phylogenetic position of H. hirame was estimated on the basis of the concatenated amino acid sequences of protein-coding genes (PCGs) from 21 other mongenean mitogenomes available in GenBank. Two Tricladida species (Crenobia alpine and Obama sp.) were used as outgroups.
The complete circular mt genome of N. hirame was 14,588 bp in size (GenBank accession no. MN984338), consisting of 12 protein-coding genes (PCGs, lacking atp8), 22 tRNA genes, 2 rRNA genes, and 1 non-coding region. The nucleotides composition was calculated at 30.0% A, 18.7% G, 10.5% C and 40.8% T. All the genes were transcribed from the same strand.
The length of 12 PCGs was 11,979 bp, occupying 82.1% of the full mt genome. Arrangement of the 12 PCGs was identical to those of all the other polyopisthocotylean monogenean mtDNA sequences published up to date: cox3-nad6-nad5-cytb-nad4l-nad4-atp6-nad2-nad1-nad3-cox1-cox2. The ATG was the start codon for all the PCGs, except for nad3 (ATA as the start codon). TAG was the stop codon in 5 PCGs (nad5, cytb, nad4l, nad4 and cox2, while TAA was the stop codon for the rest of them. The size of the 22 tRNA genes was 1,428 bp in total, ranging from 58 bp (trnS1) to 71 bp (trnE). Twenty-one of them had conventional secondary structure, but trnS1 lacked a DHU arm. The arrangement of tRNA gene was as follows: trnC-trnL1-trnY-trnS2-trnR-trnL2-trnE-trnQ-trnF-trnV-trnA-trnD-trnN-trnP-trnI-trnS1-trnW-trnG-trnT-trnK-trnM-trnH. The rrnL and rrnS were 885 bp and 732 bp in size, respectively. They were adjacent to non-coding regions next to trnK (upstream) and cox2 (downstream) and separated by a short non-coding region (10 bp). There were several non-coding regions (349 bp in total) and 1 repeat sequence (885 bp) located between trnC and nad6 genes.
Phylogenetic relationships between N. hirame and other 7 polyopisthocotyleans and 15 monopisthocotyleans were inferred from the concatenated amino acid sequences of the 12 PCGs. The tree topology obtained by MEGA X (Kumar et al. Citation2018) revealed that 2 major clades: Monopisthocotylean subclass and Polyopisthocotylean subclass. Polyopisthocotylean subclass was subsequently divided into 2 major clades: one having the Diplozoidae family (Eudiplozoon sp. DZ-2018, Sindiplozoon sp. DZ-2018, Paradiplozoon opsariichthydis) and the other having the Microcotylidae (Microcotyle sebastis, Polylabris halichoeres, Polylabroides guangdongensis), Chauhaneidae (Pseudochauhanea macrorchis), Mazocraeidae (Neomazocraes dorosomatis) and Diclidophoridae (N. hirame) (Park et al. Citation2007; Zhang et al. Citation2011, Citation2012, Citation2018) ().
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Chan PP, Lowe TM. 2019. tRNAscan-SE: searching for tRNA genes in genomic sequences. Methods Mol Biol. 1962:1–14.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549.
- Ogawa K.1999. Neoheterobothrium hirame sp. nov. (Monogenea: Diclidophoridae) from the buccal cavity wall of Japanese flounder Paralichthys olivaceus. Fish Pathol. 34(4):195–201.
- Park JK, Kim KH, Kang S, Kim W, Eom KS, Littlewood D. 2007. A common origin of complex life cycles in parasitic flatworms: evidence from the complete mitochondrial genome of Microcotyle sebastis (Monogenea: Platyhelminthes). BMC Evol Biol. 7(1):11.
- Zhang J, Wu X, Xie M, Xu X, Li A. 2011. The mitochondrial genome of Polylabris halichoeres (Monogenea: Microcotylidae). Mitochondr DNA. 22(1–2):3–5.
- Zhang J, Wu X, Xie M, Li A. 2012. The complete mitochondrial genome of Pseudochauhanea macrorchis (Monogenea: Chauhaneidae) revealed a highly repetitive region and a gene arrangement hot spot in Polyophistocotylea. Mol Biol Rep. 39(8):8115–8125.
- Zhang D, Zou H, Wu SG, Li M, Jakovlic I, Zhang J, Chen R, Li WX, Wang GT. 2018. Three new Diplozoidae mitogenomes expose unusual compositional biases within the monogenea class: implications for phylogenetic studies. BMC Evol Biol. 18(1):133.