Abstract
The species Tetrataenium yunnanense is a medicinal herb exhibiting excellent anti-tumor activity in vitro and mainly distributed in northwest Yunnan, China. The assembled genome is 142,739 base-pairs (bp) in size, with one large single-copy region of 99,975 bp and one small single-copy region of 17,375bp separated by two inverted repeats of 25,386 bp. The genome contains a total of 121 genes, including 78 protein-coding genes, 8 rRNAs and 35 tRNAs. Furthermore, phylogenomic estimation indicated that two individuals of T. yunnanense could be clustered into an obvious lineage and failed to recover the two Tetrataenium species (T. yunnanense and T. candicans) as a monophyletic group.
Tetrataenium has been widely accepted by taxonomists as a separate genus (Logacheva et al. Citation2010; Yu et al. Citation2011; Pimenov Citation2017; Xiao et al. Citation2018), but non-monophyletic, including Tetrataenium sensu stricto clade and Candicans clade (Downie et al. Citation2010; Logacheva et al. Citation2010; Yu et al. Citation2011; Liu and Downie Citation2017). Tetrataenium yunnanense (Franch.) Manden. ex Q. Y. Xiao & X. J. He. (Apiaceae, Apioideae), is a perennial herb endemic to northwest Yunnan (Dali County, Deqin County, Eryuan County, Fugong County and Gongshan County), China, growing in alpine meadows at 3200–4300 m (Xiao et al. Citation2018). In the previous researches, the species was attributed to multiple genera and described as multiple taxa, i.e. Heracleum yunnanense Franch., Angelica oncosepala Hand.-Mazz., Heracleum oncosepalum (Hand.-Mazz.) Pimenov & Kljuykov. (Franchet Citation1894; Handel-Mazzetti Citation1933; Pimenov and Kljuykov Citation2003). Heracleum yunnanense is recorded as incompletely known species only from a few collections. In the Flora of China, the roots of A. oncosepala are recorded as having reputed medicinal value (Pan and Watson Citation2005). Li et al. (Citation2012) reported further that A. oncosepala exhibit excellent anti-tumor activity in vitro and may be a good resource for new anti-tumor compounds. Here, we assembled the complete chloroplast genome of T. yunnanense to explore the reasonably systematic position and conservation of this taxon.
The mature leaves of T. yunnanense were obtained from among shrubs in grassland at coniferous forest margins Luoping Shan (26°0′56.21″N, 99°53′25.92″E, altitude 3200 m), Eryuan County, Yunnan Province, China. Voucher specimens (voucher number: xqy-2016082201) were deposited in the herbarium of the Natural History Museum of Sichuan University (SZ). The total genomic DNA was isolated from dry leaves using the TIANGEN plant genomic DNA extraction kit, following the manufacturer’s instructions and sequenced at Novogene (Novogene BioTech, Inc. Beijing, China) by Illumina Hiseq 2500 platform (Illumina, San Diego, CA). Around 5 Gb raw data were assembled against the plastome of H. yunnanense (MN365275) (Kang et al. Citation2019) and the genome obtained was annotated using software Geneious version 11.0.4 (Kearse et al. Citation2012). The annotated plastid genome sequence has been deposited into the GenBank with the accession number MN935165.
The whole plastid genome of T. yunnanense was 142,739 bp in length, with a large single-copy (LSC) region (99,975 bp), a small single-copy (SSC) region (17,375 bp), and a pair of inverted repeats (IRa and IRb: 25,386 bp). The overall GC content was 37.3%. The genome consisted of 121 genes, including 78 protein-coding genes, 35 distinct tRNA genes, and 8 rRNA genes.
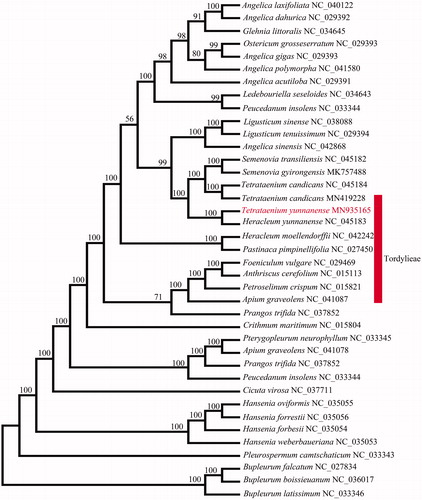
To further investigate the phylogenetic position of T. yunnanense and T. candicans, 38 cp genome sequences of Apiaceae were downloaded from the NCBI database, aligned by MAFFT (Katoh et al. Citation2017), and trimmed properly by trimAl v1.4 (Capella-Gutierrez et al. Citation2009). RAxML-HPC2 on XSEDE version 8.2.10 (Stamatakis Citation2014) was used to construct a maximum likelihood tree. As a result, phylogenetic analysis indicated that two individuals of T. yunnanense could be clustered into an obvious lineage and failed to recover the two Tetrataenium species (T. yunnanense and T. candicans) as a monophyletic group, while T. candicans is closely related to Semenovia (), supporting the separation of T. candicans from Tetrataenium sensu stricto clade to establish as a new genus (Paik Citation2008). The whole chloroplast genome sequences provided sufficient genetic information for phylogenetic reconstruction of the genus Tetrataenium.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 25(15):1972–1973.
- Downie SR, Spalik K, Katzdownie DS, Reduron JP. 2010. Major clades within Apiaceae subfamily Apioideae as inferred by phylogenetic analysis of nrDNA ITS sequences. Plant Div Evol. 128(2):111–136.
- Franchet AR. 1894. Notes sur quelques Umbelliferes du Yunan. Bull. Soc. Philom. Paris. 6:143–144.
- Handel-Mazzetti HF. 1933. Umbelliferae. In: Handel-Mazzetti HF, editor. Symbolae sinicae 7. Wien: Springer; p. 729.
- Kang L, Xie D, Xiao Q, Peng C, Yu Y, He X. 2019. Sequencing and analyses on chloroplast genomes of Tetrataenium candicans and two allies give new insights on structural variants, DNA barcoding and phylogeny in Apiaceae subfamily Apioideae. PeerJ. 7:e8063.
- Katoh K, Rozewicki J, Yamada KD. 2017. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 4:1–7.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649.
- Li Y, Chen YJ, Luo M, Yan J, Zhou YP. 2012. Anti-tumor actvivity of Chemical Constituents from Yunnan Angelica oncosepala. J Kunming Med Univ. 33(6):25–28.
- Liu M, Downie SR. 2017. The phylogenetic significance of fruit anatomical and micromorphological structures in Chinese Heracleum species and related taxa (Apiaceae). Syst Bot. 42(2):313–325.
- Logacheva MD, Valiejoroman CM, Degtjareva GV, Stratton JM, Downie SR, Samigullin TH, Pimenov MG. 2010. A comparison of nrDNA ITS and ETS loci for phylogenetic inference in the Umbelliferae: an example from tribe Tordylieae. Mol Phylogenet Evol. 57(1):471–476.
- Paik JH. 2008. Systematic studies of Heracleum L. (Umbelliferae) and related genera in the Sino-Himalayan region [doctor thesis]. Edinburgh: University of Edinburgh.
- Pan ZH, Watson MF. 2005. Angelica L. Apiaceae.In: Wu ZY and Raven PH, editors. Flora of China. Vol. 14. Beijing: Science Press; p. 164.
- Pimenov MG. 2017. Updated checklist of Chinese Umbelliferae: nomenclature, synonymy, typification, distribution. Turczaninowia. 20:106–239.
- Pimenov MG, Kljuykov EV. 2003. Notes on some SinoHimalayan species of Angelica and Ostericum (Umbelliferae). Willdenowia. 33(1):121–137.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Xiao QY, Yu Y, Xie DF, Guo XL, He XJ. 2018. Taxonomic revision of Angelica oncosepala and Heracleum yunnanense. Nord J Bot. 36(3):1–10,2018: e01563.
- Yu Y, Downie SR, He XJ, Deng XL, Yan L. 2011. Phylogeny and biogeography of Chinese Heracleum (Apiaceae tribe Tordylieae) with comments on their fruit morphology. Plant Syst Evol. 296(3–4):179–203.