Abstract
The DNA data of Indian snakes are scanty in the global database, especially from the northeastern states. The present study generated the mitochondrial Cytochrome b gene information of two morphologically identified deadly elapid species from Mizoram. Both, the King Cobra (Ophiophagus hannah) and Banded Krait (Bungarus fasciatus) showed monophyletic clades in the BA topology and cohesively clustered with the database sequences generated from distant geographical locations. The studied O. hannah depicted 2.7–7.6% K2P genetic distances with the specimens collected from China, Vietnam, and Thailand. Further, the northeast Indian B. fasciatus revealed 3.3–4% K2P genetic distance from Chinese, Vietnamese, Thailand, Indonesian, and Australian specimens. The TCS network showed distinct haplotypes for both the species collected from northeast India. The genetic information of these venomous snakes would be helpful for further rapid identification from the museum as well as from road-killed specimens, curbing the venom poaching and medical avenues.
Introduction
The legless reptiles, snakes are distributed in every continent except Antarctica with more than 3000 species till date (Wallach et al. Citation2014; Uetz and Hošek Citation2019). Among them, over 300 species are known from the Indian subcontinent of which 61 are venomous (Whitaker and Martin Citation2015). The venomous snakebites are a significant risk to human health around the world and most impacted to the rural peoples (Warrell Citation2010; WHO Citation2019). Due to the evolution of modified teeth and venom glands, these species are skilled to inject venom into the prey animals (Gong et al. Citation2010). Owing to the high mortality, disability, and priority research, the World Health Organization (WHO) enlisted the venomous snakebite as a neglected tropical disease (Warrell and WHO Citation2009). The probable number of snakebites was 5.8 million worldwide with 1.25 lakh deaths, of which over one million snakebites and 50,000 deaths occurred every year in India (Mohapatra et al. Citation2011; Dandona et al. Citation2018). In the recent past, WHO launched the snakebite envenoming guidelines with the aspiration of diminishing the number of deaths and disabilities by 50% before 2030 (Bolon et al. Citation2019; WHO Citation2019). In a medical point of view, most of the snakes are nonvenomous; however, the deadly snakes are categorized into two groups: the highly noxious, common, widespread species, and the species with restricted distribution remote from human populations (WHO Citation2010; Williams et al. Citation2019).
In India, three Elapidae species (Bungarus caeruleus, Naja kaouthia, Naja naja) and three Viperidae species (Daboia russelii, Echis carinatus, Hypnale hypnale) are grouped into first category; however, seven Elapids (Bungarus fasciatus, Bungarus niger, Bungarus sindanus, Bungarus walli, Naja oxiana, Naja sagittifera, Ophiophagus hannah) and five Vipers (Cryptelytrops albolabris, Cryptelytrops purpureomaculatus, Trimeresurus malabaricus, Trimeresurus gramineus, Macrovipera lebetina) are grouped into second category (Alirol et al. Citation2010; Menon et al. Citation2017). Nevertheless, without proper identification of species and lack of awareness, the majority of victims are immediately scared, increasing heart beat and feeling faint before medical intervention. Nevertheless, 80% of the world’s population including Indians rely on traditional medicine for snakebite envenoming and primary health-care. The rural people of India largely believe in medicinal plant-based traditional practices, juice or paste of leaves, stem barks, and roots that are applied externally or consumed orally for snakebite first aid (Goswami et al. Citation2014; Lalramnghinglova Citation2016). Apart from this, the accurate identification of biting species is crucial for appropriate treatment, especially for neurotoxic envenoming. It is often encountered that the victim does not respond to the antivenom prescribed by the medical person due to the lack of species identification (Bhattacharya and Chakraborty Citation2007). Additionally, the manufacture of antivenoms and their effectiveness against the venoms of geographical widespread snakes is still underway (Ghosh et al. Citation2016). Hence, the knowledge of indigenous species, their genetic structure, the chemistry of venom, and signs, as well as symptoms of envenomation, is crucial.
The DNA-based species identification is largely used as an effective tool for systematics research, biodiversity assessment, and forensic sciences (Hebert et al. Citation2003; Dubey et al. Citation2011; Tyagi et al. Citation2019). These molecular techniques are tremendously used to identify the snakes from widespread geographical regions (Nagy et al. Citation2012; Chambers and Hebert Citation2016). Further, the mitochondrial DNA is also evident to identify the snake species from venom samples (Singh et al. Citation2012; Supikamolseni et al. Citation2015; Sharma et al. Citation2016; Smith et al. Citation2018). Besides, the phylogeny, population structure, and evolutionary history of snakes were effectively adjudicated by the assessment of both mitochondrial and nuclear genes (Slowinski and Lawson Citation2002; Pyron et al. Citation2013; Figueroa et al. Citation2016). Hence, in the present study, we sampled two deadly Elapidae snakes from Mizoram state in northeast India. We used both morphology and mitochondrial Cytochrome b sequence information to confirm the species identity. Further, we estimated the phylogenetic tree, genetic divergence, and haplotype to determine the status of these two snakes in northeast India. The present data would further be helpful to detect the road-kill species, arresting the venom smugglers racket, and medical avenues for specific antivenom production.
Materials and methods
The field survey and sampling of King Cobra, O. hannah (23.73 N 92.67E) () and Banded Krait, B. fasciatus (24.01 N 92.37E) () was conducted by the herpetology team of Mizoram University after obtaining the permission from Chief Wildlife Warden of Environment, Forests and Climate Change, Government of Mizoram. Both the specimens were morphologically identified and vouchered at the Department of Zoology, Mizoram University, and the tissue samples were preserved in −30 °C at the Center for DNA Taxonomy laboratory, Zoological Survey of India (ZSI), Kolkata, for downstream molecular analysis. The genomic DNA isolation, PCR, purification of the PCR products, and bi-directional sequencing were performed by the standardized protocols (Kundu et al. Citation2018) . The generated sequences were checked through SeqScanner V1.0 (Applied Biosystems Inc., CA, USA), nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/), ORF finder (https://www.ncbi.nlm.nih.gov/orffinder/), and MEGAX (Kumar et al. Citation2018) to diminish the low-quality sequence read, mismatches, and gaps. The consensus sequences were contributed to the global GenBank database to acquire the accession numbers. To estimate the genetic divergence, phylogenetic analysis, and haplotyping, 13 publicly available sequences of O. hannah and 14 sequences of B. fasciatus were downloaded from GenBank with their collection locality information. Both generated and database sequences were aligned together by using ClustalX to build a dataset (Thompson et al. Citation1997). The Kimura 2 parameter (K2P) genetic distances for the studied dataset were estimated by MEGAX. The best fit model for the Bayesian analysis (BA) was calculated through Mr.Modeltest v2 with the lowest BIC value (Nylander Citation2004). The BA phylogeny was constructed in Mr. Bayes 3.1.2 by selecting nst = 6 and rates = invgamma for GTR + G + I model. The MCMC (one cold and three hot chains) was run for 1,000,000 generations with 25% burn-in and trees saving at every 100 generations along with other strand parameters (Ronquist and Huelsenbeck Citation2003). The constructed BA phylogeny was further beautified in the web-based iTOL tool (https://itol.em) (Letunic and Bork Citation2007). The database sequence of Lycodon pictus (Accession No. MN395830) under family Colubridae was used as an out-group for the present phylogenetic tree. To understand the genealogical relationships, the haplotype networks were constructed within the different populations of O. hannah and B. fasciatus. The numbers of haplotypes were generated using DnaSP v6 (Rozas et al. Citation2017). The haplotype diversity (Hd) and nucleotide diversity (p) for the different population of both the species were also calculated through DnaSP v6. The haplotype data files were further used to construct the haplotype network by using PopART (http://popart.otago.ac.nz) (Leigh and Bryant Citation2015) with the Templeton, Crandall and Sing (TCS) method (Clement et al. Citation2000).
Figure 1. (A) Live photograph of O. hannah, (B) Live photograph of B. fasciatus, (C) Bayesian phylogeny based on partial mtCytb gene inferred monophyletic clustering of both Elapidae species. (D) TCS network of O. hannah and (E) TCS network of B. fasciatus reveled distinct haplotype of both species collected from Mizoram state in northeast India. Haplotypes are shown in different color circles as represent by collection localities marked in the phylogeny.
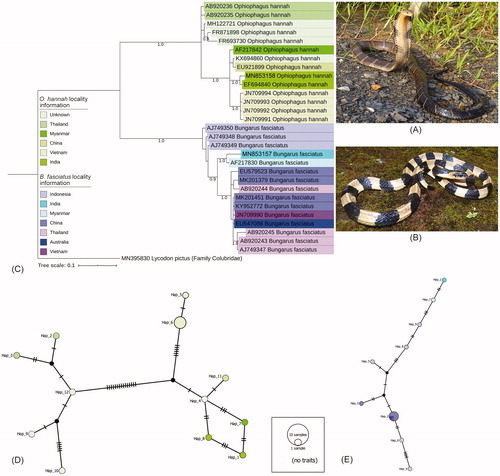
Results and discussion
The state of Mizoram considered as a part of Indo-Burma biodiversity hotspot and holds a rich assemblage of herpetofauna (ZSI Citation2007; Das Citation2010). The state houses over 60 snake species of which 11 are deadly noxious and cause 2–3 human deaths every year. These venomous snakes are predominately belonging to two families, Elapidae and Viperidae. Among them, the living Kraits are mostly active and bite during night; however, the Vipers and Cobra bite during the day time or dusk. Due to the lack of knowledge on the habitats and characteristics of extant species, snakes are facing man-made threats in this state too. Although the morphological characters readily identify this group of species, their asymmetric body size, coloration, and mimicry frequently led to the doubt in identification (Davis Rabosky et al. Citation2016). The use of molecular tools not only assist in the rapid species identification but also assure their evolutionary trends, diversification, and other genomic features to discriminate the population (Burbrink and Lawson Citation2007; Castoe et al. Citation2012). Due to the efficacy of mitochondrial Cytb gene for determining boundaries between snake species (Slowinski and Keogh Citation2000; Laopichienpong et al. Citation2016), the present study aimed to generate the molecular data of two morphologically identified Elapidae species from Mizoram state and contributed to the global databases (B. fasciatus (ZSI_SHT1): GenBank Accession No. MN853157, BOLD Process ID SKBIO001-20 and O. hannah (ZSI_SHT17): GenBank Accession No. MN853158, BOLD Process ID SKBIO002-20). Further, based on the locality information, the database sequences were merged in the analyzed dataset for estimating the intra-species genetic distances, phylogeny and haplotypes.
A total of 50 and 57 variable sites were diagnosed within the studied mitochondrial Cytb dataset (464 bp) of B. fasciatus and O. hannah, respectively. Both the Elapidae species showed 22.1% mean K2P genetic distance with each other. The within-group mean genetic distance of O. hannah was 4.2%, ranging from 0% to 7.6%. All the sequences generated from different localities were clustered together in the present BA phylogeny and a monophyletic clade was formed for this species (Figure 1C) . The studied specimen collected from Mizoram showed close clustering with the specimen (Accession No. EF694840) collected from other parts of India with 2% genetic distance; however, 0.7% genetic distance was revealed with the Burmese specimen (Accession No. AF217842). Further, the northeast Indian specimen showed 2.7% genetic distance with Chinese specimen, 4.1–4.7% genetic distance with Vietnamese specimens, and 7.6% genetic distance with Thailand specimens. The studied sequences of O. hannah resulted in 12 haplotypes with 37 polymorphic sites, haplotype diversity= 0.989. The TCS network showed a distinct haplotype of O. hannah collected from Mizoram (Figure 1D).
The within-group mean genetic distance of B. fasciatus was 3%, ranging from 0% to 8.2%. All the sequences were clustered together in the BA tree and a monophyletic clade was formed. The studied specimen collected from Mizoram showed close clustering with the specimen (Accession No. AF217830) collected from Myanmar with 1.3% genetic distance. Further, the northeast Indian specimen showed 3.3% genetic distance with the specimen collected from Thailand, Vietnam, Thailand, China, and Australia, 3.4% genetic distance with Indonesian specimens, 4% genetic distance with the specimen collected from Indonesia, China, and Thailand. Further, a single database sequence generated from Thailand depicted high genetic distance (6.1%) with the northeast Indian specimen. Based on the low genetic distances of B. fasciatus from different distant geographical localities, the present study assumed that the population of this species might have coalesced within its range distribution. The studied sequences of B. fasciatus resulted in nine haplotypes with 15 polymorphic sites, haplotype diversity = 0.885. The TCS network showed a distinct haplotype of B. fasciatus collected from Mizoram (Figure 1E).
This genetic information is not only adjudicated to the species identification and systematics studies but also triggered other ecological and biological studies of these two venomous snakes from northeast India. The in-depth study of these species will further facilitate the medical emergencies and detect the trade route of snake venom trafficking within and outside of India. We recommend a similar approach can be adopted for other deadly snakes distributed in India to reveal their genetic signature. Apart from this, the awareness among the community, capacity building and training, and improvement of medical facilities in the rural areas could diminish snakebite mortality in northeastern states and other parts of India.
Acknowledgements
We are thankful to the Director of Zoological Survey of India (ZSI), Ministry of Environment, Forest and Climate Change (MoEF&CC), Govt. of India, for providing necessary molecular facilities and support for the study. We are grateful to Michael Vanlalchhuana, Lalrinsanga, Lalmuansanga for their assistance during the field visit. The author (HTL) would like to express his sincere thanks to the Chief Wildlife Warden, Department of Environment, Forest and Climate Change, Govt. of Mizoram, for the collection permission and the DST (SERB), New Delhi for financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. 2010. Snake bite in South Asia: a review. PLOS Negl Trop Dis. 4(1):e603.
- Bhattacharya P, Chakraborty A. 2007. Neurotoxic snake bite with respiratory failure. Indian J Crit Care Med. 11(3):161–164.
- Bolon I, Finat M, Herrera M, Nickerson A, Grace D, Schütte S, Martins SB, de Castañeda RR. 2019. Snakebite in domestic animals: first global scoping review. Prev Vet Med. 170:104729.
- Burbrink FT, Lawson R. 2007. How and when did Old World ratsnakes disperse into the New World?. Mol Phylogenet Evol. 43(1):173–189.
- Castoe TA, Streicher JW, Meik JM, Ingrasci MJ, Poole AW, de Koning AP, Campbell JA, Parkinson CL, Smith EN, Pollock DD. 2012. Thousands of microsatellite loci from the venomous coralsnake Micrurus fulvius and variability of select loci across populations and related species. Mol Ecol Resour. 12(6):1105–1113.
- Chambers EA, Hebert PD. 2016. Assessing DNA barcodes for species identifcation in North American reptiles and amphibians in natural history collections. PLOS One. 11(4):e0154363.
- Clement M, Snell Q, Walker P, Posada D, Crandall K. 2000. TCS: a computer program to estimate gene genealogies. Mol Ecol. 9(10):1657–1659.
- Dandona R, Kumar GA, Kharyal A, George S, Akbar M, Dandona L. 2018. Mortality due to snakebite and other venomous animals in the Indian state of Bihar: findings from a representative mortality study. PLOS One. 13(6):e0198900.
- Das A. 2010. Systematics and biogeography of the snakes of northeast India [Thesis submitted for the degree of Doctor of Philosophy in Zoology]. Bubaneswar: Utkal University.
- Davis Rabosky AR, Cox CL, Rabosky DL, Title PO, Holmes IA, Feldman A, McGuire JA. 2016. Coral snakes predict the evolution of mimicry across new world snakes. Nat Commun. 7(1):11484.
- Dubey B, Meganathan PR, Haque I. 2011. DNA mini-barcoding: an approach for forensic identifcation of some endangered Indian snake species. Forensic Sci Int Genet. 5(3):181–184.
- Figueroa A, McKelvy AD, Grismer LL, Bell CD, Lailvaux SP. 2016. A species-level phylogeny of extant snakes with description of a new colubrid subfamily and genus. PLOS One. 11(9):e0161070.
- Ghosh S, Mukhopadhyay P, Chatterjee T. 2016. Management of snake bite in India. J Assoc Physicians Ind. 64(8):11–14.
- Gong E, Martin LD, Burnham DA, Falk AR. 2010. The birdlike raptor Sinornithosaurus was venomous. Proc Natl Acad Sci USA. 107(2):766–768.
- Goswami PK, Samant M, Srivastava RS. 2014. Snake venom, anti-snake venom and potential of snake venom. Int J Pharm Pharm Sci. 6:4–7.
- Hebert PDN, Cywinska A, Ball SL, deWaard JR. 2003. Biological identifcations through DNA barcodes. Proc R Soc Lond B. 270(1512):313–321.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549.
- Kundu S, Kumar V, Laskar BA, Tyagi K, Chandra K. 2018. Pet and turtle: DNA barcoding identified twelve geoemydid species in northeast India. Mitochondrial DNA Part B. 3(2):513–518.
- Lalramnghinglova H. 2016. Documentation of medicinal plants based on traditional practices in the Indo-Burma hotspots region of Mizoram, north east India. Emer Life Sci Res. 2:10–45.
- Laopichienpong N, Muangmai N, Supikamolseni A, Twilprawat P, Chanhome L, Suntrarachun S, Peyachoknagul S, Srikulnath K. 2016. Assessment of snake DNA barcodes based on mitochondrial COI and Cytb genes revealed multiple putative cryptic species in Thailand. Gene. 594(2):238–247.
- Leigh JW, Bryant D. 2015. popart: full-feature software for haplotype network construction. Methods Ecol Evol. 6(9):1110–1116.
- Letunic I, Bork P. 2007. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 23(1):127–128.
- Menon JC, Joseph JK, Whitaker RE. 2017. Venomous snake bite in india – why do 50,000 Indians die every year? J Assoc Physicians Ind. 65(8):78–81.
- Mohapatra B, ., Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, Rodriguez PS, Mishra K, Whitaker R, Jha P. 2011. Snakebite mortality in India: a nationally representative mortality survey. PLOS Negl Trop Dis. 5(4):e1018.
- Nagy ZT, Sonet G, Glaw F, Vences M. 2012. First large-scale dna barcoding assessment of reptiles in the biodiversity hotspot of madagascar, based on newly designed COI primers. PLOS One. 7(3):e34506.
- Nylander J. 2004. Mr.Modeltest v2, program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University.
- Pyron RA, Burbrink FT, Wiens JJ. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol. 13:93.
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19(12):1572–1574.
- Rozas J, Ferrer-Mata A, Sanchez-DelBarrio J, Guirao-Rico S, Librado P, Ramos-Onsins S, Sanchez-Gracia A. 2017. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 34(12):3299–3302.
- Sharma SK, Kuch U, Höde P, Bruhse L, Pandey DP, Ghimire A, Chappuis F, Alirol E. 2016. Use of molecular diagnostic tools for the identification of species responsible for snakebite in Nepal: a pilot study. PLOS Negl Trop Dis. 10(4):e0004620.
- Singh CS, Gaur A, Sreenivas A, Singh L. 2012. Species identifcation from dried snake venom. J Forensic Sci. 57(3):826–828.
- Slowinski JB, Keogh JS. 2000. Phylogenetic relationships of elapid snakes based on cytochrome b mtDNA sequences. Mol Phylogenetics Evol. 15(1):157–164.
- Slowinski JB, Lawson R. 2002. Snake phylogeny: evidence from nuclear and mitochondrial genes. Mol Phylogenet Evol. 24(2):194–202.
- Smith CF, McGlaughlin ME, Mackessy SP. 2018. DNA barcodes from snake venom: a broadly applicable method for extraction of DNA from snake venoms. BioTechniques. 65(6):339–345.
- Supikamolseni A, Ngaoburanawit N, Sumontha M, Chanhome L, Suntrarachun S, Peyachoknagul S, Srikulnath K. 2015. Molecular barcoding of venomous snakes and species-specifc multiplex PCR assay to identify snake groups for which antivenom is available in Thailand. Genet Mol Res. 14(4):13981–13997.
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25(24):4876–4882.
- Tyagi K, Kumar V, Kundu S, Pakrashi A, Prasad P, Caleb JTD, Chandra K. 2019. Identification of Indian spiders through DNA barcoding: cryptic species and species complex. Sci Rep. 9(1):14033.
- Uetz P, Hošek J. 2019. The reptile database. [Accessed 2019 Dec 24]. http://www.reptile-database.org.
- Wallach V, Williams KL, Boundy J. 2014. Snakes of the world: a catalogue of living and extinct species. Florida: CRC Press. Taylor and Francis Group.
- Warrell DA, WHO (World Health Organization). 2009. Neglected tropical diseases. https://www.who.int/neglected_diseases/EB132_R7_en.pdf.
- Warrell DA. 2010. Snake bite. Lancet. 375(9708):77–88.
- Whitaker R, Martin G. 2015. Diversity and distribution of medically important snakes of India. Clinical toxinology in Asia-Pacifc and Africa. Dordrecht: Springer Science+ Business Media.
- WHO (World Health Organization). 2010. venomous snakes distribution and species risk categories. https://apps.who.int/bloodproducts/snakeantivenoms/database/. Accessed 24 December 2019.
- WHO (World Health Organization) 2019. Snakebite envenoming: a strategy for prevention and control. Geneva: Licence: CC BY-NC-SA 3.0 IGO.
- Williams DJ, Faiz MA, Abela-Ridder B, Ainsworth S, Bulfone TC, Nickerson AD, Habib AG, Junghanss T, Fan HW, Turner M, et al. 2019. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLOS Negl Trop Dis. 13(2):e0007059.
- ZSI (Zoological Survey of India). 2007. Fauna of Mizoram. State Fauna Series. 14:1–691.
