Abstract
The complete mitochondrial genome sequences of Grandidierella rubroantennata and G. fasciata were determined in this study. The mitochondrial genomes were 14,469 bp and 14,656 bp in length and consisted of 37 mitochondrial genes (13 PCGs, 2 rRNAs, and 22 tRNAs). The phylogenetic analyses showed that both Grandidierella species formed one clade and were grouped with species belonging to the infraorder Corophiida, which is consistent with morphology-based classification. The results obtained in this study provide a useful reference for further phylogenetic and ecological studies.
The genus Grandidierella Coutière, 1904 is a group of amphipods inhabiting mainly in the brackish water worldwide, including more than 40 described species (Horton et al. Citation2020). Since morphology-based species identification of this genus requires considerable efforts and expert techniques, molecular characterization will help to avoid misidentification. Although the number of published amphipod mitochondrial genomes is increasing rapidly (Kumar Patra et al. Citation2019; Li et al. Citation2019, Citation2020), no complete mitogenomes has been reported for the Grandidierella genus. Here, we determined the complete mitochondrial sequences of G. rubroantennata (Ariyama and Taru Citation2017) and G. fasciata (Ariyama Citation1996).
Grandidierella rubroantennata and G. fasciata were collected from intertidal zones at Misaki-cho in Osaka, Japan (34°19′22″N, 135°06′59″E and 34°19′19″N, 135°07′12″E) on 7 April 2019. DNA was isolated using DNeasy Blood & Tissue Kit (Qiagen) and sequenced on an Illumina MiSeq with a paired-end library. The extracted DNA samples were deposited in the Radioisotope & Biotechnology Laboratory at the National Institute for Environmental Studies, Japan (NIES-202002-AMP1 and NIES-202002-AMP2). The sequence data were analyzed using CLC Genomic Workbench (ver. 12.0). The raw reads were trimmed with a Phred score < 30 after removal of adapter sequences and reads containing more than two ambiguous nucleotides. Reads shorter than 100 bases were discarded. The remaining reads were de novo assembled with the default parameters. Mitochondrial sequences were identified by similarity search using BLAST program against the nt database. The obtained mitogenomes were annotated by MITOS 2 webserver (Bernt et al. Citation2013), followed by manual curation. Phylogenetic analyses were performed based on 13 PCG sequences in the mitogenomes of two Grandidierella species and those of other 11 amphipods belonging to different superfamilies. Each amino acid sequence was aligned separately using MAFFT v7.453 with the L-INS-i option (Katoh and Standley Citation2013) and then the alignments were filtered using PREQUAL v1.02 (Whelan et al. Citation2018). Maximum likelihood phylogenetic analyses were performed using IQ-TREE 1.6.12 (Nguyen et al. Citation2015).
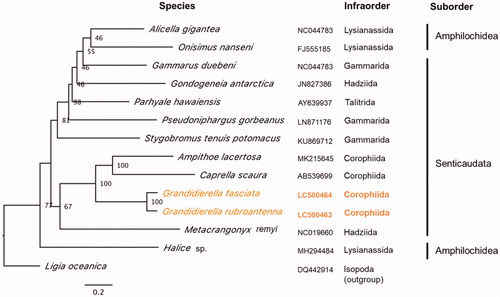
The complete circular mitogenomes of 14,469 bp and 14,656 bp were obtained for G. rubroantennata and G. fasciata (INSDC accession number: LC500463 and LC500464). The mean coverages of the mitogenomes were 72× and 73× for G. rubroantennata and G. fasciata, respectively, indicating highly confident assemblies. The mitogenomes of both species contained 13 PCGs, 2 rRNAs, and 22 tRNAs. Both species showed the same PCG order (cox1, cox2, atp8, atp6, cox3, nad3, nad6, nad5, nad4, nad4l, cytb, nad1, and nad2), but have translocations of nad6 from pancrustacean ground pattern (Boore Citation1999). PCG nucleotide sequences of the two species showed 79% identity. Phylogenetic analyses showed that both Grandidierella species formed a well-supported clade and were grouped with other two species belonging to the infraorder Corophiida (Ampithoe lacertosa and Caprella scaura) in one clade with high bootstrap value (), which is consistent with morphology-based classification by Lowry and Myers (Citation2017).
Figure 1. Maximum likelihood tree of 13 PCGs in amphipod mitochondrial genomes with their accession numbers. Species in orange represent the Grandidierella genus. Non-parametric bootstrap values (based on 200 times resampling) are shown at nodes. The phylogenetic tree was inferred using IQ-TREE v1.6.12 with the ‘–spp’ option to allow partition-specific evolution rates. The best-fit model for each PCG was determined by ModelFinder (Kalyaanamoorthy et al. Citation2017) implemented in IQ-TREE, based on Akaike information criteria. The phylogenetic tree was visualized by FigTree v1.4.4. Isopod species (Ligia oceanica) was used as an outgroup.
This is the first record of complete mitogenomes of the family Aoridae. The results of this study provide a useful reference for further phylogenetic and ecological studies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ariyama H. 1996. Four species of the genus Grandidierella (Crustacea: Amphipoda: Aoridae) from Osaka Bay and the northern part of the Kii Channel, central Japan. Publ SMBL. 37(1–2):167–191.
- Ariyama H, Taru M. 2017. Three species of Grandidierella (Crustacea: Amphipoda: Aoridae) from coastal areas of the Tohoku and Kanto-Tokai Districts, East Japan, with the description of two new species. SpecDiv. 22(2):187–200.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Boore JL. 1999. Animal mitochondrial genomes. Nucleic Acids Res. 27(8):1767–1780.
- Horton T, Lowry J, De Broyer C, Bellan-Santini D, Coleman CO, Corbari L, Costello MJ, Daneliya M, Dauvin JC, Fišer C, et al. 2020. World amphipoda database. Grandidierella Coutière. 1904 [WWW Document].
- Kalyaanamoorthy S, Minh BQ, Wong TKF, Von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–589.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Kumar Patra A, Kim MS, Jung TW, Cho IY, Yoon M, Choi JH, Yang Y. 2019. The complete mitochondrial genome of the sand-hopper Trinorchestia longiramus (Amphipoda: Talitridae). Mitochondrial DNA Part B. 4(2):2104–2105.
- Li J, Liao Y, Li J, He L. 2020. The complete mitochondrial genome of the deep- sea amphipod Eurythenes magellanicus (Crustacea: Amphipoda: Lysianassidae. Mitochondrial DNA Part B. 5(1):337–339..
- Li J, Zeng C, Yan G, He L. 2019. Characterization of the mitochondrial genome of an ancient amphipod Halice sp. MT-2017 (Pardaliscidae) from 10,908 m in the Mariana Trench. Sci Rep. 9(1):2610.
- Lowry JK, Myers AA. 2017. A phylogeny and classification of the amphipoda with the establishment of the new order Ingolfiellida (Crustacea: Peracarida). Zootaxa. 4265(1):1–89.
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Whelan S, Irisarri I, Burki F. 2018. PREQUAL: detecting non-homologous characters in sets of unaligned homologous sequences. Bioinformatics. 34:3929–3930.
