Abstract
The whole chloroplast (cp) genome sequence of Viburnum farreri has been characterized from Illumina pair-end sequencing. The complete cp genome was 158,516 bp in length, containing a large single-copy region (LSC) of 86,990 bp and a small single-copy region (SSC) of 18,502 bp, which were separated by a pair of inverted repeat (IR) regions of 26,512 bp. The genome contained 130 genes, including 85 protein-coding genes, 37 tRNA genes, and 8 ribosomal RNA genes (4 rRNA species). Most genes occur as a single copy, while 17 gene species are duplicated. Phylogenetic analysis revealed that V. farreri is closely related to the species of V. brachybotryum.
Viburnum, the basal-most lineage in the Adoxaceae family (Jacobs et al. Citation2010), contains approximately 200 species, distributed from South America to South East Asia (Lobstein et al. Citation1999). Extensive research suggests that there are flavonoids, bioflavonoids, and coumarins in different Viburnum species (Cometa et al. Citation1998). Viburnum farreri is a large deciduous shrub in Viburnum which is commonly cultivated in botanical gardens around the world because of its high ornamental value. To date, there are many researches on the phylogenetic placement of Viburnum (Donoghue et al. Citation2004; Donoghue and Winkworth Citation2005; Bibi et al. Citation2010; Clement et al. Citation2014), however, there are very few studies on the V. farreri. In this study, we assembled the complete chloroplast genome of V. farreri which can be useful for the evolution and genetics research of the genus Viburnum in future.
In this study, fresh leaves of V. farreri were sampled from Xianyang in Shaanxi Province, China (N34°16′06″, E108°04′29″). Meanwhile, a voucher specimen was deposited in the Herbarium of Guizhou Education University (No. SX10309). Genomic DNA was extracted from the fresh leaves using the CTAB method (Doyle Citation1987). Total DNA was used for the shotgun library construction and the subsequent high-throughput sequencing on the Illumina HiSeq 2500 Sequencing System. In total, 2.3 G raw reads were obtained, quality-trimmed, and used for the cp genome assembly using MITObim v1.8 (Hahn et al. Citation2013) with V. Japonicum (GenBank: MH036493.1) (Cho et al. Citation2018) as the initial reference. The genome was visualized and annotated in Geneious version 9.0.2 (Biomatters Ltd., Auckland, New Zealand). A neighbor-joining (NJ) tree was inferred using MEGA6.0 (Tamura et al. Citation2013) from alignments created by the MAFFT (Katoh and Standley Citation2013) using 10 other complete chloroplast genomes previously reported in Adoxaceae. The annotated genomic sequence has been submitted to GenBank with the accession number MT133259.
The circular chloroplast genome of V. farreri was 158,516 bp in size, and comprises a pair of inverted repeat (IR) regions of 26,512 bp each, a large single-copy (LSC) region of 86,990 bp, and a small single-copy (SSC) region of 18,502 bp. The chloroplast genome contained 130 genes including 85 protein-coding genes, 37 tRNA genes, 8 rRNA genes. In these genes, 18 genes contained one intron and two genes contained two introns. The majority of the gene species are single copy; however, 17 gene species in the IR regions are totally duplicated, including 6 protein-coding genes, 7 tRNA genes and 4 rRNA genes. Out of these 17 gene species, rps12 are partially located within the IR regions, while all the others completely within the IR regions. The overall GC content of V. farreri chloroplast genome is 38.1%.
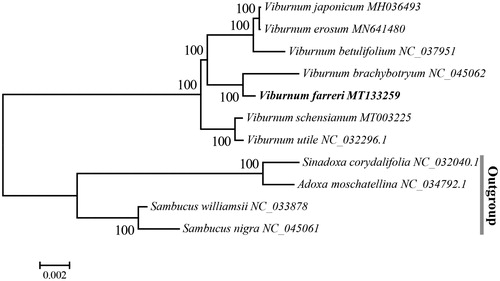
To investigate its taxonomic status of V. farreri, 11 complete chloroplast genomes were selected to construct a neighbour-joining tree using MEGA software version 6.0 and bootstrap analysis of 1000 replicates of which Sinadoxa corydalifolia, Adoxa moschatellina, Sambucus williamsii and Sambucus nigra were used as outgroup. The NJ phylogenetic tree shows that the seven species in Viburnum were clustered together, V. farreri is most related to V. brachybotryum with bootstrap support values of 100% (). Our complete cp genome data of V. farreri may contribute to a better understanding of the evolution of Viburnum.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bibi Y, Nisa S, Waheed A, Zia M, Sarwar S, Ahmed S, Chaudhary MF. 2010. Evaluation of Viburnum foetens for anticancer and antibacterial potential and phytochemical analysis. Afr J Biotechnol. 9(34):5611–5615.
- Cho WB, Han EK, Choi HJ, Lee JH. 2018. The complete chloroplast genome sequence of Viburnum japonicum (Adoxaceae), an evergreen broad-leaved shrub. Mitochondrial DNA Part B. 3(1):458–459.
- Clement WL, Arakaki M, Sweeney PW, Edwards EJ, Donoghue MJ. 2014. A chloroplast tree for viburnum (Adoxaceae) and its implications for phylogenetic classification and character evolution. Am J Bot. 101(6):1029–1049.
- Cometa MF, Mazzanti G, Tomassini L. 1998. Sedative and spasmolytic effects of Viburnum tinus L. and its major pure compounds. Phytother Res. 12(S1):S89–S591.
- Donoghue MJ, Baldwin BG, Li J, Winkworth RC. 2004. Viburnum phylogeny based on chloroplast trnk intron and nuclear ribosomal its DNA sequences. Syst Bot. 29(1):188–198.
- Donoghue MJ, Winkworth RC. 2005. Viburnum phylogeny based on combined molecular data: implications for taxonomy and biogeography. Am J Bot. 92(4):653–666.
- Doyle JJ. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Hahn C, Bachmann L, Chevreux B. 2013. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads – a baiting and iterative mapping approach. Nucleic Acids Res. 41(13):e129–e129.
- Jacobs B, Huysmans S, Smets E. 2010. Evolution and systematic value of fruit and seed characters in Adoxaceae (Dipsacales). Taxon. 59(3):850–866.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Lobstein A, Haan-Archipoff G, Englert J, Kuhry JG, Anton R. 1999. Chemotaxonomical investigation in the genus Viburnum. Phytochemistry (Oxford). 50(7):1175–1180.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30(12):2725–2729.