Abstract
Cartilaginous fishes are a highly vulnerable vertebrate group but remain poorly studied, especially those occupying deep-water ecological niches. Here, we describe the complete mitogenome of the deep-water chimaeriform Hydrolagus affinis (de Brito Capello, 1868) (Holocephali: Chimaeridae). The mitogenome has 19,437 nucleotides and the same overall content, i.e. 13 protein-coding genes, 22 transfer RNA, 2 ribosomal RNA genes, as available for all cartilaginous fishes mitogenomes. Phylogenetic reconstructions including 615 cartilaginous fishes mitogenomes place the H. affinis within the family Chimaeridae but suggest that Hydrolagus and Chimera are not reciprocally monophyletic, highlighting the need for additional molecular data to improve phylogenetic reconstruction.
Research into Chondrichthyes Huxley, 1880 (cartilaginous fishes) biology is critical to understand the evolution of vertebrates. Their ecology, physiology, and evolutionary placement are decisive to understand the origin and evolution of gnathostomes (Inoue et al. Citation2010; Renz et al. Citation2013; Boisvert et al. Citation2019). The subclass Holocephali (chimaeras, ratfishes, and rabbitfishes) is composed of three families (Callorhinchidae Garman, 1901, Rhinochimaeridae Garman, 1901, and Chimaeridae Bonaparte, 1831) widely distributed in the oceans, usually found below 200 m and up to 2000 m (Didier et al. Citation2012). Their deep-water habitat poses many challenges, resulting in a generalized lack of taxonomic, biological, ecological, and evolutionary knowledge (Venkatesh et al. Citation2014; Boisvert et al. Citation2019). Chimaeridae includes nearly 70% of known Holocephalan and comprises two genera, Chimaera Linnaeus, 1758 and Hydrolagus Gill, 1862.
Application of molecular approaches, e.g. mitochondrial genomes (mtDNA), allowed a more comprehensive and detailed biodiversity assessment of cartilaginous fishes (Arnason et al. Citation2001; Inoue et al. Citation2010; Johri et al. Citation2019a, Citation2019b). Yet, and especially for holocephalans, most studies are still based on morphology or single mitochondrial markers (e.g. Didier et al. Citation2012; Walovich et al. Citation2017). Only 10 complete mtDNA have been sequenced for Holocephali; therefore, increasing taxonomic representation is essential to improve our understanding of their evolutionary relationships.
A male Hydrolagus affinis (de Brito Capello, 1868) specimen was captured in the Canadian North-Atlantic (47.3685 N; 46.6540 W) during the EU Groundfish Survey (Fletán Negro 3L-2018). Morphological identification was performed on board and later confirmed by COI mtDNA. Genomic DNA was extracted and used for whole-genome sequencing of 150 bp paired-end (PE) reads on Hiseq X Ten (BioProject PRJNA606208).
Mitogenome assembly was obtained using NOVOPlasty (v.3.7.1) (Dierckxsens et al. Citation2016) with 10% of the PE reads (SRR11071609) and annotated using MITOS2 (Bernt et al. Citation2013).
All available (614) mitogenomes of cartilaginous fishes were retrieved from GenBank (01 November 2019) and their 13 protein-coding genes (PCGs) aligned and concatenated using MAFFT (v.7.402) on XSEDE (Katoh and Standley Citation2013) and SequenceMatrix (v.1.7.8) (Vaidya et al. Citation2011), respectively (final length: 11,405 bp). The best partition-scheme for each gene was estimated using PartitionFinder2 (v.2.1.1) on XSEDE (Lanfear et al. Citation2016) and used for Bayesian phylogeny (GTR + I + G) in MrBayes (v.3.2.6), on XSEDE (Ronquist et al. Citation2012) with two independent runs (107 generations, sampling frequency one tree for 1000 generations) and maximum likelihood (ML) estimations on RAxML (v.8.2.12) (Stamatakis Citation2014) HPC Black Box, with a ‘halt bootstrapping automatically’ and 20 ML searches. All analyses were implemented in CIPRES (Miller et al. Citation2010). Genetic sequence divergence (uncorrected p-distance) was calculated using MEGAX (Kumar et al. Citation2018).
The new mitogenome was deposited in GenBank with accession number MT090368 (BioProject PRJNA606208), with a length of 19,437 bp, within the expected range for Holocephalan (16,758–24,889 bp). Gene content and orientation are expected for vertebrate mtDNA: 13 PCGs, 22 transfer RNA, and 2 ribosomal RNA genes. Only one PCG (NAD6) and eight tRNAs are encoded on the complementary strand.
Congruent BI and ML phylogenetic trees, rooted at the split between Holocephali and the remaining cartilaginous fishes (), recovered the two subclasses as reciprocally monophyletic, i.e. Holocephali and Elasmobranchii (Boisvert et al. Citation2019).
Figure 1. Bayesian inference phylogenetic tree based on 615 cartilaginous fishes mitogenomes sequences of 13 concatenated protein coding genes. GenBank accession numbers for Holocephalan taxa are behind species names. *Both posterior probabilities and bootstrap support values above 99%.
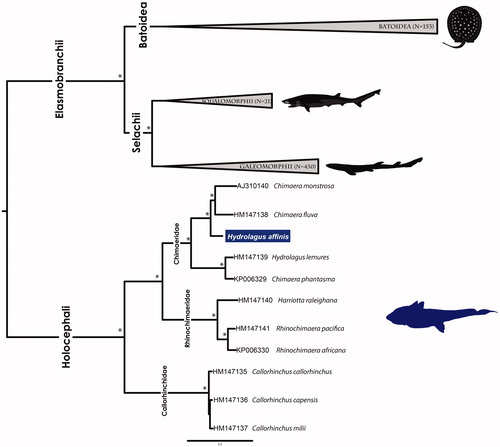
In Holocephali, three well-supported clades were identified, Callorhinchidae, Rhinochimaeridae, and Chimaeridae, supporting the most comprehensive Holocephalan phylogenetic reconstructions (Arnason et al. Citation2001; Inoue et al. Citation2010). Although Hydrolagus and Chimaera cluster within the Chimaeridae group (), neither genus was recovered as monophyletic. The two Hydrolagus species show a sequence divergence of 14%. Hydrolagus affinis clusters together with C. monstrosa and C. fluva, showing between 10% and 9% p-distances. Conversely, H. lemures clusters with C. phantasma, showing only 5% p-distance. Together these results reinforce the need for multidisciplinary approaches to further clarify Holocephali systematics and taxonomy.
Acknowledgements
We thank all the participants and crew of the cruise Demersales19 performed on board the R/V Miguel Oliver.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arnason U, Gullberg A, Janke A. 2001. Molecular phylogenetics of gnathostomous (jawed) fishes: old bones, new cartilage. Zool Scripta. 30(4):249–255.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Boisvert CA, Johnston P, Trinajstic K, Johanson Z. 2019. Chondrichthyan evolution, diversity, and senses. Cham: Springer.
- Didier AD, Kemper MJ, Ebert AD. 2012. Phylogeny, biology, and classification of extant Holocephalans. In: Carrier J, Musick J, Heithaus M, editors. Biology of sharks and their relatives. Boca Raton: CRC Press; p. 97–122. http://www.crcnetbase.com/doi/abs/10.1201/b11867-11.
- Dierckxsens N, Mardulyn P, Smits G. 2016. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45:e18.
- Inoue JG, Miya M, Lam K, Tay BH, Danks JA, Bell J, Walker TI, Venkatesh B. 2010. Evolutionary origin and phylogeny of the modern Holocephalans (Chondrichthyes: Chimaeriformes): a mitogenomic perspective. Mol Biol Evol. 27(11):2576–2586.
- Johri S, Doane M, Allen L, Dinsdale E. 2019a. Taking advantage of the genomics revolution for monitoring and conservation of Chondrichthyan populations. Diversity. 11(4):49.
- Johri S, Solanki J, Cantu VA, Fellows SR, Edwards RA, Moreno I, Vyas A, Dinsdale EA. 2019b. Genome skimming’ with the MinION hand-held sequencer identifies CITES-listed shark species in India’s exports market. Sci Rep. 9(1):4476.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549.
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2016. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 34:772–773.
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. 2010 Gateway Computing Environments Workshop, GCE 2010. IEEE. p. 1–8. http://ieeexplore.ieee.org/document/5676129/.
- Renz AJ, Meyer A, Kuraku S. 2013. Revealing less derived nature of cartilaginous fish genomes with their evolutionary time scale inferred with nuclear genes. PLOS One. 8(6):e66400.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Vaidya G, Lohman DJ, Meier R. 2011. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics. 27(2):171–180.
- Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, Ohta Y, Flajnik MF, Sutoh Y, Kasahara M, et al. 2014. Elephant shark genome provides unique insights into gnathostome evolution. Nature. 505(7482):174–179.
- Walovich KA, Ebert DA, Kemper JM. 2017. Hydrolagus erithacus sp. Nov. (Chimaeriformes: Chimaeridae), a new species of chimaerid from the south-eastern Atlantic and Southwestern Indian oceans. Zootaxa. 4226(4):509–520.
