Abstract
Pistia stratiotes is an invasive aquatic weed in South China. In this study, the first complete chloroplast (cp) genome of P. stratiotes was reported and phylogenetic analysis was conducted with Araceae species based on the cp genome sequences. The genome is a circular molecule of 164,551 bp in length with 36.00% average GC content and includes a large single-copy region (90,705 bp), a small single-copy region (21,886 bp), and two inverted repeat regions (25,980 bp). It contains a total of 129 genes, including 84 protein-coding genes, 37 tRNA genes, and 8 rRNA genes. The maximum likelihood tree indicated that P. stratiotes is related to the genus of Alocasia. The cp genome will provide useful molecular data for further phylogenetic and evolutionary analysis of P. stratiotes.
Pistia stratiotes L., commonly known as water lettuce, is the only surviving species of Pistia genus in Araceae. It is a perennial, free-floating aquatic plant which originated in the Tethys region (Renner and Zhang Citation2004). It was introduced in China during the 16th century. After naturalization, P. stratiotes has become a problematic weed and now it is on the second shortlist of alien invasive species in China and widely distributed in tropical and subtropical countries around the world (Liu et al. Citation2017). In most natural situations, the species grows rapidly and forms dense mats on surface of water bodies, disrupts native aquatic flora and fauna underneath, threats to biodiversity and impedes fishing, water sport, boat traffic and flood control (Khan et al. Citation2014; Eid Citation2017). On the other hand, P. stratiotes has been shown to have great potential in phytoremediation due to its ability to accumulate pollutants from wastewater, such as heavy metals (Putra et al. Citation2015), pestcides (Chattoraj et al. Citation2014), oils (Yang et al. Citation2014), pharmaceuticals and personal care products (Lin and Li Citation2016). To further understand its genetic background, here we assembled and characterized the complete chloroplastt (cp) genome sequence of P. stratiotes.
Fresh leaf samples of P. stratiotes were collected from Baiyun Lake (23°06′32″N, 113°15′53″E), Guangzhou, Guangdong province of China. The specimen was deposited in the herbarium of Guangzhou City Polytechnic (voucher number: GCP0648). High-quality genomic DNA of P. stratiotes was extracted from leaves by TIANGEN plant genomic DNA kit and sequenced by Novaseq platform (Illumina, San Diego, CA, USA). Approximately, 9 Gb of raw data of 150 bp paired-end reads were generated and assembled using GetOrganelle (Jin et al. Citation2019). Genes and the corresponding coding regions were annotated with Geseq (Tillich et al. Citation2017). Finally, the validated complete cp genome sequence of P. stratiotes was submitted to GenBank with accession number MN885890.
The complete cp genome of P. stratiotes is 164,551 bp in length with a typical quadripartite structure consisting of a large single-copy (LSC) region of 90,705 bp, a small single-copy (SSC) region of 21,886 bp, and a pair inverted repeat (IR) regions of 25,980 bp. A total of 129 genes were predicted, including 84 protein-coding genes, 37 tRNA genes, and 8 rRNA genes. The overall GC content of the genome is 36.00%. The GC content of three regions is ranked as 55.12, 15.79, and 13.30% for LSC, IR, and SSC, respectively.
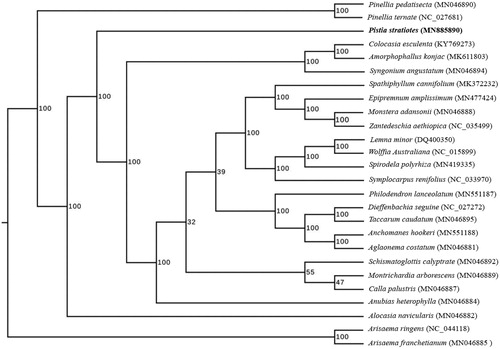
In order to confirm the phylogenetic position of P. stratiotes, a maximum likelihood analysis was performed by RAxML (Stamatakis Citation2014) with 1000 bootstrap replicates (Minh et al. Citation2013; Chernomor et al. Citation2016) based on 26 complete cp genomes of Araceae, including the typical species of all genera currently registered in GenBank. As shown in , P. stratiotes is related to the genus of Alocasia, consistent with previous studies (Cusimano et al. Citation2011). The finding will provide valuable molecular data for further phylogenetic and evolutionary analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in National Center for Biotechnology Information (NCBI) at https://www.ncbi.nlm.nih.gov/, accession number MN885890.
Additional information
Funding
References
- Chattoraj S, Mondal NK, Das B, Roy P, Sadhukhan B. 2014. Biosorption of carbaryl from aqueous solution onto Pistia stratiotes biomass. Appl Water Sci. 4(1):79–88.
- Chernomor O, von Haeseler A, Minh BQ. 2016. Terrace aware data structure for phylogenomic inference from supermatrices. Syst Biol. 65(6):997–1008.
- Cusimano N, Bogner J, Mayo SJ, Boyce PC, Wong SY, Hesse M, Hetterscheid WLA, Keating RC, French JC. 2011. Relationships within the Araceae: comparison of morphological patterns with molecular phylogenies. Am J Bot. 98(4):654–668.
- Eid ME. 2017. Verification of a numerical growth model of Pistia stratiotes L. using field data from tropical and subtropical sites. J Freshwater Ecol. 32(1):391–403.
- Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, Li DZ. 2019. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. bioRxiv, doi:10.1101/256479.
- Khan MA, Marwat KB, Gul B, Wahid F, Khan H, Hashim S. 2014. Pistia stratiotes L. (Araceae): phytochemistry, use in medicines, phytoremediation, biogas and management options. Pak J Bot. 46(3):851–860.
- Lin YL, Li BK. 2016. Removal of pharmaceuticals and personal care products by Eichhornia crassipe and Pistias tratiotes. J Taiwan Inst Chem E. 58:318–323.
- Liu DS, Wang R, Gordon DR, Sun XH, Chen L, Wang YW. 2017. Predicting plant invasions following China's Water Diversion Project. Environ Sci Technol. 51(3):1450–1457.
- Minh BQ, Nguyen MAT, von Haeseler A. 2013. Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol. 30(5):1188–1195.
- Putra RS, Cahyana F, Novarita D. 2015. Removal of lead and copper from contaminated water using EAPR system and uptake by water lettuce (Pistia Stratiotes L.). Procedia Chem. 14:381–386.
- Renner SS, Zhang LB. 2004. Biogeography of the Pistia Clade (Araceae): based on chloroplast and mitochondrial DNA sequences and bayesian divergence time inference. Syst Biol. 53(3):422–432.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq – versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–W11.
- Yang XN, Chen SS, Zhang RD. 2014. Utilization of two invasive free-floating aquatic plants (Pistia stratiotes and Eichhornia crassipes) as sorbents for oil removal. Environ Sci Pollut Res Int. 21(1):781–786.