Abstract
We sequenced the complete mitochondrial genome of Schoutedenia ralumensis. The mitogenome is 16,051 bp long with an A + T content of 84.5%, including 13 protein-coding genes, 22 transfer RNA genes, 2 ribosomal RNA genes, a control region, and an aphid-specific repeat region located between trnE and trnF. All protein-coding genes are initiated by ATN and terminated with TAA or TAG except for cox1 and nad5. All transfer RNAs display the typical clover-leaf secondary structure except for trnS (AGN). The unique repeat region is 974 bp long, in which a 305-bp repeat unit repeats 3.19 times. The phylogenetic tree supports a sister relationship of S. ralumensis and Greenidea psidii.
Keywords:
Schoutedenia ralumensis Rübsaamen, 1905 is an aphid species widely distributed in southeastern Asia, India, Africa and Australia. It feeds on the plants of Euphorbiaceae, with a monoecious and partially holocyclic life cycle (Blackman and Eastop Citation2020). In this study, using Illumina sequencing, we characterize the complete mitochondrial genome of S. ralumensis, the first representative from the aphid tribe Schoutedeniini (Aphididae: Greenideinae). The aphid samples were collected on Breynia fruticosa from Mt. Wuzhi, Hainan, China (18.9045°N, 109.6820°E) and deposited in the National Zoological Museum of China, Institute of Zoology, Chinese Academy of Sciences, Beijing, China (NZMC no. 24309).
The S. ralumensis mitogenome is 16,051 bp long (GenBank accession number MT381994), which comprises 13 protein-coding genes, 22 transfer RNA genes (tRNAs), 2 ribosomal RNA genes (rRNAs), a control region, and a repeat region located between trnE and trnF. All 37 genes are arranged in the same order as the inferred ancestral arrangement of insects (Clary and Wolstenholme Citation1985). The overall nucleotide composition is 38.9% T, 9.8% C, 45.6% A and 5.7% G, with an A + T content of 84.5%. All protein-coding genes use the typical ATN start codons and TAA or TAG stop codons except for cox1 and nad5, which end with a single T. The tRNA genes range from 62 to 73 bp in length. All tRNAs exhibit a typical clover-leaf secondary structure except for trnS (AGN), which losses the dihydrouridine (DHU) arm. The rrnL and rrnS genes are 1267 and 767 bp in length, with an A + T content of 86.2% and 84.9%, respectively.
The control region located between rrnS and trnI is 542 bp long with an A + T content of 84.2%. The unique repeat region between trnE and trnF, which has been found in previously reported mitogenomes of Greenideinae species (i.e. Greenidea psidii van der Goot and Greenidea ficicola Takahashi) (Chen et al. Citation2019; Liu et al. Citation2020), is also present in the mitogenome of S. ralumensis. It is 974 bp in length with an A + T content of 85.5%. A 305-bp repeat unit repeats 3.19 times in this region.
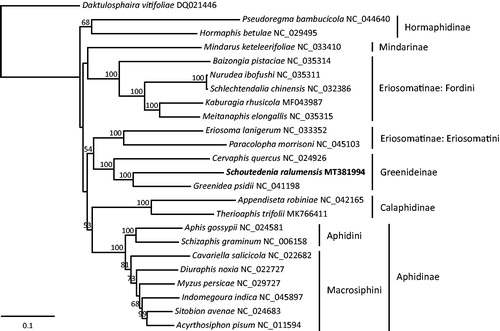
Based on the whole mitochondrial genome sequences of S. ralumensis and 23 other aphid species, we constructed a maximum-likelihood phylogenetic tree of aphids using RAxML v8.2.10 (Stamatakis Citation2014). The Greenideinae was recovered as a monophyletic clade with strong support and S. ralumensis was firmly placed as a sister to G. psidii (). The subfamilies Hormaphidinae, Calaphidinae and Aphidinae were all monophyletic, whereas the Eriosomatinae was polyphyletic.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in Dryad at https://doi.org/10.5061/dryad.dv41ns1vg.
Additional information
Funding
References
- Blackman RL, Eastop VF. 2020. Aphids on the world’s plants; [accessed 2020 Apr 25]. http://www.aphidsonworldsplants.info/
- Chen J, Wang Y, Qin M, Jiang LY, Qiao GX. 2019. The mitochondrial genome of Greenidea psidii van der Goot (Hemiptera: Aphididae: Greenideinae) and comparisons with other Aphididae aphids. Int J Biol Macromol. 122:824–832.
- Clary DO, Wolstenholme DR. 1985. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 22(3):252–271.
- Liu Q, Zhang H, Deng J, Lin X, Hang X. 2020. The complete mitochondrial genome of Greenidea ficicola (Hemiptera: Aphididae: Greenideinae), a pest of Ficus. Mitochondrial DNA Part B. 5(1):254–256.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.