Abstract
Ampelocalamus scandens is native to Guizhou Province, China, and grows at an altitude of 260–320 m. It can be used as a raw material for weaving and papermaking. In the current study, the complete chloroplast (cp) genome of A. scandens was sequenced and is reported for the first time. The complete cp sequence was 139,504 bp, include large single-copy (LSC), small single-copy (SSC), and a pair of invert repeats (IR) region of 83,103 bp, 12,813 bp, and 21,793 bp, respectively. Besides, the plastid genome comprised a total of 132 genes, including protein-coding, tRNA, and rRNA genes as 85, 39, and 8 genes, respectively. Phylogenetic analysis based on 28 cp genomes reveals that A. scandens is closely associated with Ampelocalamus melicoideus in Arundinarodae.
Ampelocalamus scanden is a vine-like bamboo with a pole length of up to 10 m. It is a unique bamboo species native to Guizhou Province, China (Geng and Wang Citation1996). A. scandens is listed as threatened species in the International Union for Conservation of Nature (IUCN) red list in 2007, with an evaluation of Critically Endangered species. Therefore, there is a need to protect and expand its distribution (Meng et al. Citation2012). In this study, we reported the complete chloroplast genome (cp) of A. scandens based on Illumina pair-end sequencing data. Fresh leaves samples of A. scandens were collected from Fujian province, China (Fujian Agriculture and Forestry University, Bamboo Garden, Fuzhou; located at 26°5′7″N, 119°14′16″E, and dried into silica gel instantly. The specimen were kept in the Herbarium of College of Forestry, Fujian Agriculture and Forestry University (code HTY006). DNA was extracted from fresh leaves tissue, with 500 bp randomly interrupted sequence by the Covaris ultrasonic breaker for library construction. The constructed library was sequenced PE150 by Illumina Hiseq Xten platform, with ∼2GB data generated. Illumina data were filtered by a script in the cluster (default parameter: -L5, -p0.5, -N0.1). The complete plastid genome of Arundinaria faberi (GeneBank accession: JX513414) as a reference and plastid genome of A. scandens were assembled by GetOrganelle pipe-line (https://github.com/Kinggerm/GetOrganelle), which can get the plastid-like reads, and these were viewed and edited by Bandage (Wick et al. Citation2015). The cp genome annotation was assembled based on the comparison by Geneious v 11.1.5 (Biomatters Ltd, Auckland, New Zealand) (Kearse et al. Citation2012). The annotation result was drawn with the online tool OGDRAW (http://ogdraw.mpimp-golm.mpg.de/) (Lohse et al. Citation2013).
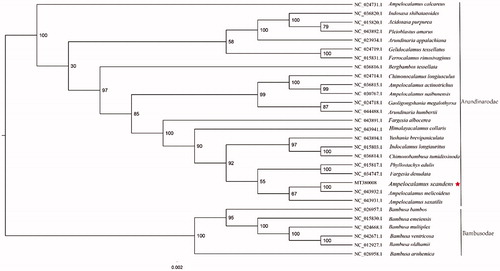
The complete plastid genome sequence of A. scandens (GenBank accession:MT380008) was 139,504 bp in length, with a large single-copy (LSC) region of 83,103 bp, a small single-copy (SSC) region of 12,813 bp, and a pair of inverted repeat (IR) regions of 21,793 bp. The complete chloroplastid genome contains 132 genes, having 85 protein-coding genes, 39 tRNA genes, and 8 rRNA genes. The complete genome GC contents were 38.9%. To reveal the phylogenetic position of A. scandens with other members of Arundinarodae, a phylogenetic analysis was performed based on 22 complete chloroplast genomes of Arundinarodae, and six taxa (Bambusa bambos, Bambusa emeiensis, Bambusa multiplex, Bambusa ventricosa, Bambusa oldhamii, Bambusa arnhemica) as outgroups; downloaded from NCBI GenBank. The sequences were aligned by MAFFT v7.307 (Katoh and Standley Citation2013), and the phylogenetic tree was constructed by RAxML (Stamatakis Citation2014) The phylogenetic tree showed that A. scandens was close to its confamilial plant, Ampelocalamus melicoideus ().
Acknowledgments
The author thanks anonymous reviewers who provided helpful suggestions and critical comments on this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are openly available in https://www.ncbi.nlm.nih.gov/ GeneBank with following accession number MT380008.
Additional information
Funding
References
- Geng BJ, Wang ZP. 1996. Compilation of “Volume of Chinese Flora” nine volumes and one volume (Gramineae-Bamboo Subfamily). J Bamboo Res. 9(1):77–79.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649.
- Lohse M, Drechsel O, Kahlau S, Bock R. 2013. OrganellarGenomeDRAW – a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 41(Web Server issue):W575–W581.
- Meng WY, Gou GQ, He LJ, et al. 2012. Research on the tissue culture of endemic bamboo in Guizhou. J Mountain Agri Biol. 31(02):116–118.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Wick RR, Schultz MB, Zobel J, Holt KE. 2015. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics. 31(20):3350–3352.