Abstract
The Arma custos is an important natural enemy of agricultural and forest pests. The complete mitochondrial genome (mitogenome) of A. custos was determined in the present paper. This mitogenome is 15,629 bp in size and comprises of 13 protein-coding genes, 22 transfer RNA genes, two ribosomal RNA genes, and a control region. All protein-coding genes are initiate with ATN, except ND2, COX1, ATP8 and ND1 use TTA or TTG as the start codon, and terminate with TAA with the exception of COX2 and ND5 which use a single T residue as the stop codon. All tRNAs, ranging from 63 to 72 bp, have the cloverleaf structure except tRNASer(AGN). The monophyly of the subfamily Asopinae is highly supported by the phylogenetic tree and A. custos is recovered as sister to the remaining Asopinae species.
Pentatomidae is one of the most diverse groups in Heteroptera. This family currently contains more than 4,949 species distributed in 940 genera and ten subfamilies, occurring in the Neotropical region exclusively (Rider et al. Citation2018). In Pentatomidae, the subfamily Asopinae is a group of predators that present great potential for biological control (De Clercq Citation2000; Lupoli Citation2019). Arma custos has been used as predators because of its ability to effectively suppress a wide range of agricultural and forest pests (Zou et al. Citation2013; Zhao et al. Citation2018). In this study, the complete mitochondrial genome (mitogenome) of A. custos was sequenced and described. The sample was collected from Yongzhou, Hunan, China (26°21′16″N 111°11′39″E). Voucher specimen is stored at the Entomological Museum of China Agricultural University (No. HEM-047).
The complete mitogenome of A. custos is 15,629 bp long including 37 typical insect mitochondrial genes (13 protein-coding genes, 22 transfer RNA genes, and two ribosomal RNA genes) and a control region. No rearrangement occurs in this mitogenome and gene order is identical to the putative ancestral arrangement of insects (Cameron Citation2014; Xu et al. Citation2020). Except for the control region, 19 inter-genic regions, ranging from 1 to 27 bp, have been found in this mitogenome. There are totally 33 bp overlapped nucleotides between neighboring genes in 7 locations, ranging from 1 to 8 bp in size.
The A + T content of the mitogenome is 75.8% (A = 42.1%, T = 33.7%, C = 13.7%, G = 10.5%) which is significantly biased toward AT. The AT-skew is positive (0.11) whereas GC-skew is negative (−0.13). The nucleotide skew statistics for the minority strand (N strand) exhibited moderate T skew and G skew, and the majority strand (J strand) display a slight T skew and C skew. Nine protein-coding genes initiate with ATN codons (3 with ATA, 4 with ATG, and 2 with ATT), whereas ND2 start with TTA and TTG is used by COX2, ATP8 and ND1 as the start codon, which were also reported common in other true bugs (Wang et al. Citation2017). The stop codon TAA was assigned to 11 protein-coding genes. The exceptions are the COX2 and ND5 use a single T residue as incomplete stop codon which is common in true bug mitogenomes (Zhao et al. Citation2017; Zhang et al. Citation2019).
There are 22 tRNA genes, ranging from 63 to 72 bp in length, in this mitogenome determined by the tRNAscan-SE (Lowe and Chan Citation2016) and ARWEN (Laslett and Canbäck Citation2008). The secondary structure of 21 tRNAs were typical clover-leaf structure except the tRNASer(AGN), in which the dihydrouridine (DHU) arm formed a loop, as is common phenomenon in most insects (Jiang et al. Citation2016). The length of lrRNA and srRNA is 1,285 bp and 797 bp, respectively. The A + T content of lrRNA and srRNA are 79.1% and 77.4% which are higher than the whole genome. The control region is located between srRNA and tRNAIle, which is 923 bp in length with an A + T content of 74.1%.
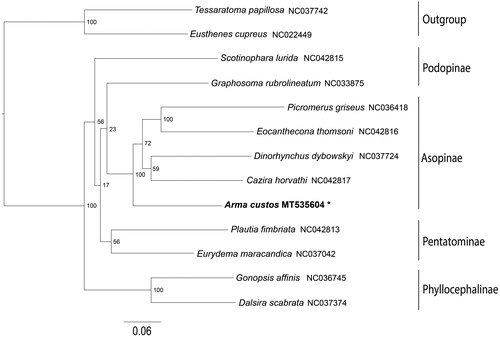
Phylogenetic tree was constructed by maximum-likelihood (ML) analysis and generated by IQ-TREE 1.6.5 (Trifinopoulos et al. Citation2016), based on the dataset of the 13 protein-coding genes and two rRNA genes from 11 species in Pentatomidae and two outgroups (). Each subfamily showed a monophyletic cluster except the Podopinae, since Graphosoma rubrolineatum presented a sister position to Asopinae. This result indicate that the subfamily Podopinae may be a paraphyly group, which is congruent with previous hypotheses (Liu et al. Citation2019). The monophyly of the Asopinae is highly supported in this phylogenetic analysis and A. custos is recovered as sister to the remaining Asopinae species. The mitogenome information of A. custos could provide basic data for the future studies of the mitogenomic diversities and evolution of the true bugs.
Disclosure statement
All authors have read and approved the final manuscript. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Data availability statement
The data that support the findings of this study will be available in GenBank at https://www.ncbi.nlm.nih.gov/, Accession number MT535604.
Additional information
Funding
References
- Cameron SL. 2014. Insect mitochondrial genomics: implications for evolution and phylogeny. Annu Rev Entomol. 59:95–117.
- De Clercq P. 2000. Predaceous stinkbugs (Pentatomidae: Asopinae). In: Schaefer CW, Panizzi AR, editors, Heteroptera of economic importance. Cambridge: Cambridge University Press; p. 737–789.
- Jiang P, Li H, Song F, Cai Y, Wang JY, Liu J, Cai WZ. 2016. Duplication and remolding of tRNA genes in the mitochondrial genome of Reduvius tenebrosus (Hemiptera: Reduviidae). IJMS. 17(6):951.
- Laslett D, Canbäck B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24(2):172–175.
- Liu YQ, Li H, Song F, Zhao YS, Wilson JJ, Cai WZ. 2019. Higher-level phylogeny and evolutionary history of Pentatomomorpha (Hemiptera: Heteroptera) inferred from mitochondrial genome sequences. Syst Entomol. 44(4):810–819.
- Lowe TM, Chan PP. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 44(W1):W54–W57.
- Lupoli R. 2019. First catalogue of the Asopinae (Hemiptera, Pentatomidae) from French Guiana. Zootaxa. 4668(1):zootaxa.4668.1.4–88.
- Rider DA, Schwertner CF, Vilímová J, Rédei D, Kment P, Thomas DB. 2018. Higher systematics of the Pentatomoidea. In: McPherson JE, editor. Invasive stink bugs and related species (Pentatomoidea). Boca Raton (FL): CRC Press; p. 26–201.
- Trifinopoulos J, Nguyen LT, Haeseler AV, Minh BQ. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44(W1):W232–W235.
- Wang J, Zhang L, Yang XZ, Zhou MQ, Yuan ML. 2017. The first mitochondrial genome for the subfamily Podopinae (Hemiptera: Pentatomidae) and its phylogenetic implications. Mitochondrial DNA B. 2(1):219–220.
- Xu S, Wu Y, Cai W, Song F. 2020. The complete mitochondrial genome of the lychee stinkbug Mattiphus splendidus (Hemiptera: Tessaratomidae). Mitochondrial DNA B. 5(1):321–322.
- Zhang DL, Gao J, Li M, Yuan J, Liang J, Yang H, Bu W. 2019. The complete mitochondrial genome of Tetraphleps aterrimus (Hemiptera: Anthocoridae): Genomic comparisons and phylogenetic analysis of Cimicomorpha. Int J Biol Macromol. 130:369–377.
- Zhao Q, Wei J, Bu W, Liu G, Zhang H. 2018. Synonymize Arma chinensis as Arma custos based on morphological, molecular and geographical data. Zootaxa. 4455(1):161–176.
- Zhao W, Zhao Q, Li M, Wei J, Zhang X, Zhang H. 2017. Characterization of the complete mitochondrial genome and phylogenetic implications for Eurydema maracandica (Hemiptera: Pentatomidae). Mitochondrial DNA B. 2(2):550–551.
- Zou DY, Wu HH, Coudron TA, Zhang LS, Wang MQ, Liu CX, Chen HY. 2013. A meridic diet for continuous rearing of Arma chinensis (Hemiptera: Pentatomidae: Asopinae). Biol Control. 67(3):491–497.