Abstract
The complete mitochondrial genome of Pentapodus setosus which belongs to the family Nemipteridae was first determined. The complete mitochondrial genome was 16,836 bp in length with 37 genes, including 13 protein-coding genes, 22 tRNA genes, 2 rRNA genes, and a control region. Phylogenetic analysis using mitochondrial genomes of 11 related species revealed that P. setosus formed a well-supported monophyletic group with the other Nemipteridae species. This mitochondrial genome provides a useful information for resolving the taxonomic issues.
The Butterfly whiptail, Pentapodus setosus (Spariformes, Nemipteridae), is a reef-associated marine fish widely distributed in the Western Central Pacific including the Philippines, South China sea, Singapore, and Indonesia (Russell Citation1990). The taxonomic position of the Nemipteridae have long been controversial. Although previous phylogenetic studies showed the position of Nemipteridae within the order Spariformes (Johnson Citation1981; Carpenter and Johnson Citation2002; Sanciangco et al. Citation2016), the position of this family remains unclear. In this study, we reported the complete mitochondrial genome sequence of P. setosus and phylogenetic analysis.
The P. setosus specimen was collected from Ho Chi Minh City, Vietnam (10.53N, 106.45W). Total genomic DNA was extracted from the specimen tissue, which has been deposited at the National Marine Biodiversity Institute of Korea (Voucher No. MABIK0002434). The mitogenome was sequenced using Illumina Hiseq 4000 sequencing platform (Illumina, San Diego, CA) and assembled with SOAPdenovo at Macrogen Inc. (Korea). The complete mitochondrial genome was annotated using MacClade ver. 4.08 (http://macclade.org/macclade; Maddison and Maddison, Citation2005) and tRNAscan-SE ver. 2.0 (http://lowelab.ucsc.edu/tRNAscan-SE; Lowe and Chan Citation2016).
The complete mitochondrial genome of P. setosus (GenBank accession no. LC557138) is 16,836 bp in length and includes 13 protein-coding genes, 22 tRNA genes, 2 rRNA genes, and a control region. The overall base composition is 28.83% A, 27.24% C, 16.49% G, and 27.44% T. All tRNA genes can fold into a typical cloverleaf structure, with lengths ranging from 68 to 74 bp. The 12S rRNA (979 bp) and 16S rRNA genes (1730 bp) are located between tRNAPhe and tRNAVal and between tRNAVal and tRNALeu(UUR), respectively. Of the 13 protein-coding genes, 12 start with ATG; the exception being COI, which starts with GTG. The stop codon of the protein-coding genes is TAA (ND1, COI, ATP8, ND4L, and ND5), T (COII, ND3, ND4, and Cytb), TA (ND2, ATP6, and COIII), and TAG (ND6). A control region (1107 bp) is located between tRNAPro and tRNAPhe.
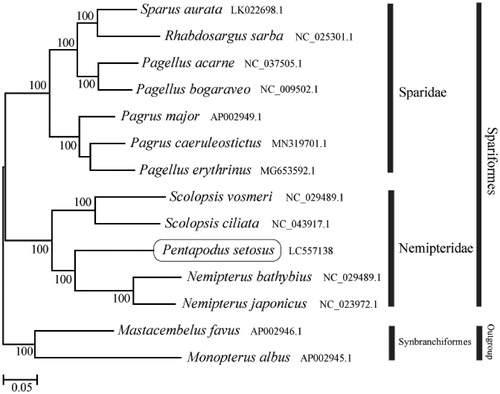
The phylogenetic trees were constructed by the maximum-likelihood method using MEGA 7.0 software (MEGA, Philadelphia, PA; Kumar et al. Citation2016). We analyzed the phylogenetic trees of the newly sequenced genome and 11 other complete Nemipteridae and Sparidae species mitochondrial genome sequences acquired from the National Center for Biotechnology Information. We confirmed that P. setosus formed a monophyletic group with the other Nemipteridae species (). This mitochondrial genome in this study provides an important resource for the phylogeny and evolution analysis.
Disclosure statement
The authors report no conflict of interests. The authors alone are responsible for the content and writing of the paper.
Data availability statement
The data that support the findings of this study are openly available in the DNA Data Bank of Japan (accession no. LC557138) at https://www.ddbj.nig.ac.jp.
Additional information
Funding
References
- Carpenter KE, Johnson GD. 2002. A phylogeny of sparoid fishes (Perciformes, Percoidei) based on morphology. Ichthyol Res. 49(2):114–127.
- Johnson GD. 1980. The limits and relationships of the Lutjanidae and associated families. In: Bulletin of the Scripps institution of oceanography of the University of California. Vol. 24. p. 1–114.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874.
- Lowe TM, Chan PP. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 44(W1):W54–W57.
- Maddison DR, Maddison WP. 2005. MacClade 4: analysis of phylogeny and character evolution. Ver. 4.08. Sunderland (MA): Sinauer Associates.
- Russell BC. 1990. Nemipterid fishes of the world (Threadfin breams, whiptail breams, monocle breams, dwarf monocle breams, and coral breams). Family Nemipteridae. An annotated and illustrated catalogue of Nemipterid species known to date. FAO Fisheries Synopsis. Vol. 12, No. 125. Rome: Food and Agriculture Organization of the United States; p. 89–90.
- Sanciangco MD, Carpenter KE, Betancur-R R. 2016. Phylogenetic placement of enigmatic percomorph families (Teleostei: Percomorphaceae). Mol Phylogenet Evol. 94(Pt B):565–576.