Abstract
Synsepalum dulcificum D. belongs to the Sapotaceae family, which is an evergreen shrub native to tropical West Africa. It is a kind of magical plant that has the unique characteristic of modifying sour flavors to sweet. In this study, the chloroplast genome of S. dulcificum was sequenced, assembled, and annotated firstly. Chloroplast genome size of S. dulcificum is 158,463 bp, the circular chloroplast genome consists of four regions: a large single-copy region of 88,256 bp, two inverted repeat regions of 25,958 bp, and a small single-copy region of 18,669 bp, with the GC content of 36.87%. A total of 133 genes were annotated in the S. dulcificum chloroplast genome, of which 88 were protein-coding genes (PCGs), 37 were transfer RNA (tRNA) genes, and eight were ribosomal RNA (rRNA) genes. Phylogenetic analysis showed that Pouteria campechiana was most closely related to S. dulcificum. The study provides important genomic data for further utilization and breeding of S. dulcificum.
Synsepalum dulcificum D. belongs to the Sapotaceae family (Shi et al. Citation2016), commonly known as miracle berry, miracle fruit, magic fruit, miraculous or asaba (Coronel et al. Citation2009; Obafemi et al. Citation2017), which is an evergreen shrub native to tropical West Africa (Wang et al. Citation2011). It is a kind of magical plant that has the unique characteristic of modifying sour flavors to sweet (Du et al. Citation2014). It is also a well-known natural source of ‘miraculin,’ a sweetening glycoprotein (Tchokponhoué et al. Citation2019). All plant parts of S. dulcificum are of medicinal importance. The berry fruit and leaf contains many nutrients along with many beneficial characteristics (Nkwocha et al. Citation2014; Njoku et al. Citation2015). It has the ability to improve insulin sensitivity, and antioxidant and anticancer abilities (Swamy et al. Citation2014). Thus, it may be used as an adjuvant for treating diabetic patients with insulin resistance (Chen et al. Citation2006; Obafemi et al. Citation2017). The seed oil of S. dulcificum is also a rare and exotic fruit oil (Guney and Nawar Citation1977).
As a valuable plant species, currently, S. dulcificum is utilized in cosmetics and food. Besides, it is also used extensively in the pharmaceutical industry (Achigan-Dako et al. Citation2015). Surprisingly, there are few studies on the genomics of S. dulcificum, focusing on the nuclear, mitochondrial and chloroplast genome, which severely limits its utilization and breeding. In this study, the chloroplast genome of S. dulcificum was sequenced, assembled, and annotated firstly.
Young leaves of S. dulcificum were collected from the Xishuangbanna Tropical Flowers and Plants Garden (100.789433 E, 22.015939 N) and frozen in liquid nitrogen. The genomic DNA was extracted using the Dneasy Plant Mini Kit (Qiagen) and then stored in the ultra-low temperature specimen library at YITC (specimen accession number: YITC-2020-FZ-S-013). DNA was sequenced using the Illumina Hiseq 2500 Platform (Illumina, San Diego, CA). The complete chloroplast genome was assembled with CLC Genomics Workbench v3.6 (http://www.clcbio.com) and annotated with Dual Organelle GenoMe Annotator (DOGMA; Wyman et al. Citation2004). The chloroplast genome was submitted to GenBank (http://www.ncbi.nlm.nih.gov/) under the accession number MT723946, and a physical map was generated by OGDRAW (http://ogdraw.mpimp-golm.mpg.de/) (Lohse et al. Citation2013).
Chloroplast genome size of S. dulcificum is 158,463 bp. Like other plants, the circular chloroplast genome consists of four regions: a large single-copy region of 88,256 bp, two inverted repeat regions of 25,958 bp, and a small single-copy region of 18,669 bp. The composition of the four bases is 49,395 bp of A, 50,640 bp of T, 28,594 bp of G, and 29,834 bp of C, with the GC content of 36.87%. There are 88 protein-coding genes (PCGs), eight ribosomal RNA (rRNA) genes, and 37 transfer RNA (tRNA) genes, with a total number of 133 genes that were annotated in the S. dulcificum chloroplast genome.
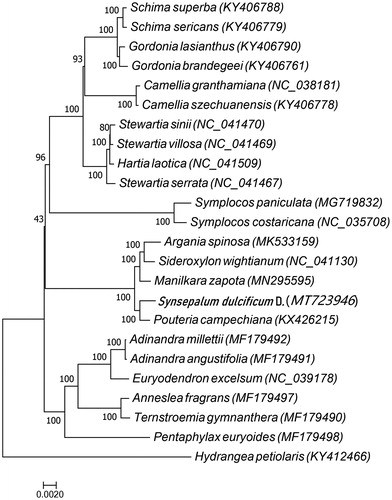
A maximum-likelihood (ML) tree based on the sequences of protein-coding genes of S. dulcificum and other 23 plant species was constructed to verify its phylogenetic relationship. The names of 70 genes common to all species which were used to construct the phylogenetic tree are as follows: Ndhb, Rps12, Rps11, Petn, Ndhf, Rps16, Ndhd, Rps14, Ndhj, Ndhk, Rps18, Rpl2, Ycf3, Rps7, Psab, Rps4, Rps3, Rps2, Rpl22, Rps8, Petd, Petg, Peta, Rps15, Petb, Rpoc1, Petl, Ycf4, Rpoc2, Ycf2, Rpl20, Rpl23, Rpob, Atpi, Atph, Atpb, Atpa, Ndhg, Atpf, Atpe, Psbd, Psba, Psaj, Psbc, Psbb, Psbm, Ndhc, Psbn, Psbi, Psbh, Psbk, Clpp, Psbt, Rpl16, Ndhh, Rpoa, Rpl14, Rpl36, Rpl33, Infa, Psaa, Ndhi, Psai, Ndha, Rbcl, Ndhe, Ccsa, Matk, Cema, and Psac. Multiple sequence alignment was done with MAFFT (Katoh and Standley Citation2013) and ML analysis was done with MEGA7.0 (Kumar et al. Citation2016). The 24 plant species included in the phylogenetic tree have been derived from the following families: Theaceae (10 species), Symplocaceae (two species), Sapotaceae (five species) and Pentaphylacaceae (six species), and Hydrangea petiolaris which belongs to Hydrangeaceae family and was used as the out group. The result showed that S. dulcificum was most closely related to Pouteria campechiana (). The study provides important genomic data for further utilization and breeding of S. dulcificum.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov/, reference number MT723946.
Figure 1. Maximum-likelihood tree based on the sequences of protein-coding genes of 24 plant species, include 10 species of Theaceae family, two species of Symplocaceae family, five species of Sapotaceae family, and six species of Pentaphylacaceae, and Hydrangea petiolaris which belongs to Hydrangeaceae family was used as the outgroup. The species and chloroplast genome accession numbers for the phylogenetic tree construction are Schima superba (KY406788), Schima sericans (KY406779), Gordonia lasianthus (KY406790), Gordonia brandegeei (KY406761), Camellia szechuanensis (KY406778), Camellia granthamiana (NC_038181), Stewartia sinii (NC_041470), Stewartia villosa (NC_041469), Hartia laotica (NC_041509), Stewartia serrata (NC_041467), Symplocos paniculata (MG719832), Symplocos costaricana (NC_035708), Argania spinosa (MK533159), Sideroxylon wightianum (NC_041130), Manilkara zapota (MN295595), Synsepalum dulcificum D. (MT723946), Pouteria campechiana (KX426215), Adinandra millettii (MF179492), Adinandra angustifolia (MF179491), Euryodendron excelsum (NC_039178), Ternstroemia gymnanthera (MF179490), Anneslea fragrans (MF179497), Pentaphylax euryoides (MF179498), and Hydrangea petiolaris (KY412466).
Additional information
Funding
References
- Achigan-Dako EG, Tchokponhoué DA, N’Danikou S, Gebauer J, Vodouhè RS. 2015. Current knowledge and breeding perspectives for the miracle plant Synsepalum dulcificum (Schum. et Thonn.) Daniell. Genet Resour Crop Evol. 62(3):465–476.
- Chen CC, Liu IM, Cheng JT. 2006. Improvement of insulin resistance by miracle fruit (Synsepalum dulcificum) in fructose-rich chow-fed rats. Phytother Res. 20(11):987–992.
- Coronel RE, Sotto RC, Rabara RC. 2009. The dwarf and round-fruited miracle fruit [Synsepalum dulcificum (Schum. & Thonne) Daniell]. Crop Protection Newsletter. 34(3):108–111.
- Du L, Shen Y, Zhang X, Prinyawiwatkul W, Xu ZM. 2014. Antioxidant-rich phytochemicals in miracle berry (Synsepalum dulcificum) and antioxidant activity of its extracts. Food Chem. 153(15):279–284.
- Guney S, Nawar WW. 1977. Seed lipids of the miracle fruit (Synsepalum Dulcificum). J Food Biochem 1(2):173–184.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger Datasets. Mol Biol Evol. 33(7):1870–1874.
- Lohse M, Drechsel O, Kahlau S, Bock R. 2013. Organellar GenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 41:575–581.
- Njoku NE, Ubbaonu CN, Alagbaoso SO, Eluchie CN, Umelo MC. 2015. Amino acid profile and oxidizable vitamin content of Synsepalum dulcificum berry (miracle fruit) pulp. Food Sci Nutr. 3(3):252–256.
- Nkwocha C, Njoku ON, Ekwueme FN. 2014. Phytochemical, antinutrient and amino acid composition of Synsepalum dulcificum pulp. IOSR J Pharm Biol Sci. 9(2):25–29.
- Obafemi TO, Akinmoladun AC, Olaleye MT, Agboade SO, Onasanya AA. 2017. Antidiabetic potential of methanolic and flavonoid-rich leaf extracts of Synsepalum dulcificum in type 2 diabetic rats. Journal of Ayurveda and Integrative Medicine. 8(4):238–246.
- Shi YC, Lin KS, Jhai YF, Lee BH, Han YF, Cui ZB, Hsu WH, Wu SC. 2016. Miracle fruit (Synsepalum dulcificum) exhibits as a novel anti-hyperuricaemia agent. Molecules. 21(2):140.
- Swamy KB, Hadi SA, Sekaran M, Pichika MR. 2014. The clinical effects of Synsepalum dulcificum: a review. J Med Food. 17(11):1165–1169.
- Tchokponhoué DA, N'Danikou S, Houéto JS, Achigan-Dako EG. 2019. Shade and nutrient-mediated phenotypic plasticity in the miracle plant Synsepalum dulcificum (Schumach. & Thonn.) Daniell. Sci Rep. 9(1):5135.
- Wang HM, Chou YT, Hong ZL, Chen HA, Chang YC, Yang WL, Chang HC, Mai CT, Chen CY. 2011. Bioconstituents from stems of Synsepalum dulcificum Daniell (Sapotaceae) inhibit human melanoma proliferation, reduce mushroom tyrosinase activity and have antioxidant properties. J Taiwan Inst Chem Eng. 42(2):204–211.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20(17):3252–3255.
