Abstract
In this study, the mitochondrial genome of Mileewa margheritae (Hemiptera: Cicadellidae: Mileewinae) was sequenced and annotated. The mitogenome of M. margheritae is 15376 bp in length, containing 13 protein-coding genes (PCGs), 22 tRNA genes, two rRNA genes, and a control region. The A + T content in the mitogenome was 79%. Most of PCGs started with ATN and stopped with TAA while ATP8 and ND5 started with TTG, COX2, ND1 stopped with incomplete T, Cytb stopped with TAG. We further constructed a Bayesian phylogenetic tree among M. margheritae, M. albovittata and other Cicadellidae species. The constructed phylogenetic tree suggests the close evolutionary relationship between M. margheritae and M. albovittata.
The genus Mileewa was established by Distant (Citation1908), which belongs to tribe Mileewini, the largest and most widely distributed tribe of Mileewinae (Krishnankutty and Dietrich Citation2011). The genus Mileewa now included 49 species in China (Yang et al. Citation2017) and widely distributed. This study further enriched mitogenome database of the tribe Mileewini.
Two male adult of M. margheritae was selected as specimen, which was collected from Huaping, Guilin, Guanngxi, China (109°56′30″E, 25°36′16.4″N), in May 2017. The genomic DNA was extracted from the entire body without abdomen by using Qiagen DNeasy Blood and Tissue Kit. The male genitalia were deposited in the Institute of Entomology, Guizhou University, Guiyang, China (GUGC), with the deposited number GUGC-IDT-00520. The mitogenome of M. margheritae was sequenced by Illumina NovaSeq6000 platform (Berry Genomics, Beijing, China). The reads were assembled by MitoZ v2.3 (Meng et al. Citation2019) then annotated by using Geneious Prime (v2020.1.2) and MITOS2 webserver. All tRNA genes were identified by ARWEN v1.2 (Laslett and Canbäck Citation2008). Annotated sequence of M. margheritae mitogenome was submitted to GenBank with accession number MT483998.
The mitogenome of M. margheritae is 15376 bp in size included 37 typical genes (13 PCGs, 22 tRNA genes and 2 rRNA genes), and a control region. The A + T content is 79%, which is similar to M. albovittata (He et al. Citation2019). Eleven PCGs have ATN as the start codon, except for ATP8 and ND5 genes started with TTG. Most PCGs used TAA as stop codon while COX2, ND1 stopped with incomplete T, and Cytb stopped with TAG. The 16S rRNA and 12S rRNA of rRNA gene are 1,214bp and 836 bp, respectively. The length of 22 tRNA is range from 62 bp (tRNA-C) to 72 bp (tRNA-K).
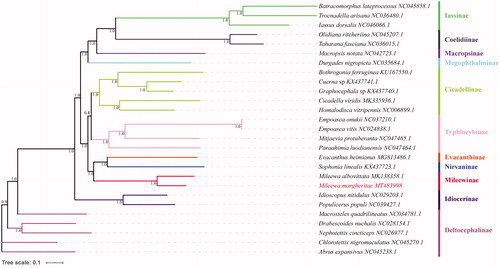
Phylogenetic relationship was constructed on nucleotides sequences of 13 PCGs among M. margheritae and 27 reference species from family Cicadellidae. The nucleotide sequences of all 13 PCGs genes for each of the above species were extracted from GenBank files using PhyloSuite (Zhang et al. Citation2020), then 13 sequences were aligned in batches with MAFFT (Katoh and Standley Citation2013) using ‘–auto’ strategy and codon alignment mode. Ambiguously aligned fragments of 13 alignments were removed in batches using Gblocks (Talavera and Castresana Citation2007). Best partitioning scheme and evolutionary models for 13 pre-defined partitions were selected using PartitionFinder2 (Lanfear et al. Citation2017), with greedy algorithm and AICc criterion. Bayesian Inference phylogenies were inferred using MrBayes 3.2.6 (Ronquist et al. Citation2012) under partition model (2 parallel runs, 65900 generations), in which the initial 25% of sampled data were discarded as burn-in. The phylogenetic tree showed that M. margheritae was clustered with M. albovittata, most of species from same subfamilies were all clustered into same clade, respectively (). The result is consistent with the traditional taxonomy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in [NCBI] at [https://www.ncbi.nlm.nih.gov/], reference number [KU167550.1, KX437723.1, KX437740.1, KX437741.1, MG813486.1, MK138358.1, MK335936.1, MT483998, NC_006899.1, NC_024838.1, NC_026977.1, NC_028154.1, NC_029203.1, NC_034781.1, NC_035684.1, NC_036015.1, NC_036480.1, NC_037210.1, NC_039427.1, NC_042723.1, NC_045207.1, NC_045238.1, NC_045270.1, NC_045858.1, NC_046066.1, NC_047464.1, NC_047465.1].
Additional information
Funding
References
- Distant WL. 1908. The Fauna of British India including Ceylon and Burma. Rhynchota. 4:238.
- He HL, Li HX, Yang MF. 2019. Complete mitochondrial genome sequence of Mileewa albovittata (Hemiptera: Cicadellidae: Mileewinae). Mitochondrial DNA Part B Res. 4(1):740–741.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Krishnankutty SM, Dietrich CH. 2011. Review of Mileewine Leafhoppers (Hemiptera: Cicadellidae: Mileewinae) in Madagascar, with description of seven new species. Ann Entom Soc Amer. 104(4):636–648.
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2017. Partitionfinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 34(3):772–773.
- Laslett D, Canbäck B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinform. 24(2):172–175.
- Meng GL, Li YY, Yang CT, Liu SL. 2019. Mitoz: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 47(11):e63–e63.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 56(4):564–577.
- Yang MF, Meng ZH, Li ZZ. 2017. Hemiptera: Cicadellidae (II): Cicadellinae. Fauna Sinica: Insecta, vol. 67. Beijing, China: Science Press.
- Zhang D, Gao F, Jakovlic I, Zou H, Zhang J, Li WX, Wang GT. 2020. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 20(1):348–355.