Abstract
The African leaf butterfly Kallimoides rumia Doubleday, 1849 (Nymphalidae), lives in the understory of Afrotropical primary forests. Genome skimming with Illumina sequencing of K. rumia permitted assembly of a complete circular mitogenome of 15,234 bp consisting of 80.9% AT nucleotides, 22 tRNAs, 13 protein-coding genes, 2 rRNAs and a control region. Kallimoides rumia COX1 features an atypical start codon (CGA). Genes ATP6, COX1, COX2, ND2, ND4 and ND5 feature incomplete stop codons, completed by adding 3’ A residues to the mRNA. Phylogenetic reconstruction places K. rumia as a distinct lineage, not closely related to Kallima, consistent with previous phylogenetic hypotheses.
The African leaf butterfly, Kallimoides rumia, is native to the understory of Afrotropical primary forests (Nyafwono et al. Citation2014). Shirôzu and Nakanishi (Citation1984) reclassified K. rumia from the leaf-mimicking Asian butterfly genus Kallima Doubleday (1849) into the monotypic genus Kallimoides based on morphology, but further phylogenetic placement within subfamily Nymphalinae has been uncertain (Larsen Citation2005). More recent molecular phylogenetic work places Kallimoides as sister to the monotypic African genus Vanessula (Wahlberg et al. Citation2009). Here we report the complete mitochondrial genome sequence of K. rumia from specimen Krum 2016.1, collected in Abiak Owo, Nigeria (GPS 4.689 N, 8.267E) in June 2016, that was pinned, spread and deposited in the Wallis Roughley Museum of Entomology, University of Manitoba (voucher WRME050773).
DNA was prepared from a specimen leg using a DNEasy Blood and Tissue kit (Qiagen, Düsseldorf, Germany) with slight modifications to the standard protocol as described in McCullagh and Marcus (Citation2015). DNA was sheared by sonication and a fragment library was prepared as previously described (Peters and Marcus Citation2017), before sequencing by Illumina NovaSeq6000 (San Diego, California) (Marcus Citation2018). Mitogenome assembly of K. rumia (Genbank accession MT704827) we performed in Geneious 10.1.2, by mapping the resulting sequence library to a Junonia stygia (Lepidoptera: Nymphalidae) reference mitogenome (MN623383) (Living Prairie Mitogenomics Consortium Citation2020) using 5 iterations of the medium sensitivity settings of Geneious 10.1.2. The mitogenome was assembled from 11,311,871 paired 150 bp reads. Annotation was performed by aligning the Kallimoides mitogenome with annotated mitogenomes from J. stygia, Precis andremiaja (MH917706) (Lalonde and Marcus Citation2019a), and Salamis anteva (MH917707) (Lalonde and Marcus Citation2019b), in Geneious 10.1.2 and protein coding genes were identified by sequence homology. The K. rumia nuclear rRNA repeat (MT704830) was also assembled and annotated using similar methods and a J. stygia rRNA reference sequence (MN623382) (Living Prairie Mitogenomics Consortium Citation2020).
The K. rumia circular 15,234 bp mitogenome assembly composition was of 175,724 paired reads with nucleotide composition: 40.1% A, 11.6% C, 7.5% G, and 40.8% T. The gene composition and order in K. rumia is identical to all known butterfly mitogenomes (Cao et al. Citation2012; McCullagh and Marcus Citation2015). Kallimoides rumia COX1 features an atypical CGA start codon as in many other insects (Liao et al. Citation2010). The mitogenome contains five protein-coding genes (COX1, COX2, ND2, ND4, ND5) with single-nucleotide (T) stop codons, and one protein-coding gene (ATP6) with a two-nucleotide (TA) stop codon completed by post-transcriptional addition of 3′ A residues. The locations and structural determination of tRNAs used ARWEN v.1.2 (Laslett and Canback Citation2008). tRNAs have typical cloverleaf secondary structures except for trnS (AGN) where a loop replaced the dihydrouridine arm. The mitochondrial rRNAs and control region are typical for Lepidoptera (McCullagh and Marcus Citation2015).
Phylogenetic reconstruction used complete mitogenomes from K. rumia, and 38 additional mitogenomes from tribes Junoniini, Kallimini, Nymphalini, and outgroup Melitaeini within subfamily Nymphalinae (Hamilton et al. Citation2020; Lalonde and Marcus Citation2019a, Citation2019b; Lalonde and Marcus Citation2020; McCullagh and Marcus Citation2015; Peters and Marcus Citation2017). Mitogenome sequences were aligned in CLUSTAL Omega (Sievers et al. Citation2011) using the default settings. The aligned sequences were then analyzed by parsimony and maximum likelihood searches performed in PAUP* 4.0b8/4.0d78 (Swofford Citation2002) (). For the maximum likelihood analysis, a GTR + G optimal model (G = 0.2250) was identified by jModeltest 2.1.7 software (Darriba et al. Citation2012), followed by a likelihood ratio test (Huelsenbeck and Rannala Citation1997).
Figure 1. Maximum likelihood phylogeny (GTR + G model, G = 0.2250, likelihood score 105306.54771) of Kallimoides rumia and 38 additional mitogenomes from subfamily Nymphalinae based on 1 million random addition heuristic search replicates (with tree bisection and reconnection). One million maximum parsimony heuristic search replicates produced 8 trees (parsimony score 17652 steps) which differ from one another only by the arrangement of Junonia coenia mitogenomes and one of which has an identical tree topology to the maximum likelihood tree depicted here. Numbers above each node are maximum likelihood bootstrap values and numbers below each node are maximum parsimony bootstrap values (each from 1 million random fast addition search replicates).
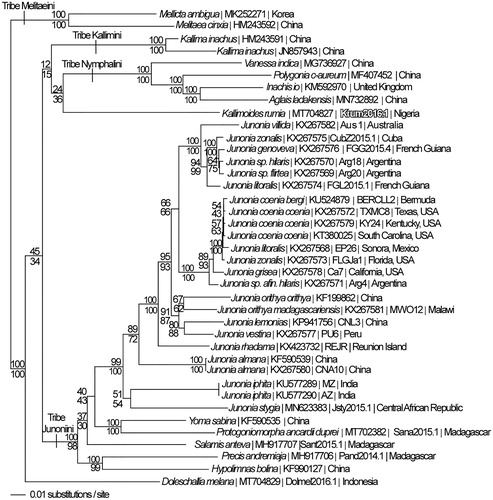
Phylogenetic analysis places K. rumia within its own monotypic genus and supports the hypothesis of Shirôzu and Nakanishi (Citation1984) that separated Kallimoides from Kallima. Hypotheses grouping Kallimoides with Vanessula could not be addressed due to lack of mitogenome data from Vanessula (Kodandaramaiah and Wahlberg Citation2007; Wahlberg et al. Citation2005).
Acknowledgments
We thank Mackenzie Alexiuk, and Rayna Hamilton for their constructive criticism on this manuscript and Genome Quebec for assistance with library preparation and sequencing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov, reference numbers MT704827 and MT704830.
Additional information
Funding
References
- Cao YQ, Ma C, Chen JY, Yang DR. 2012. The complete mitochondrial genomes of two ghost moths, Thitarodes renzhiensis and Thitarodes yunnanensis: the ancestral gene arrangement in Lepidoptera. BMC Genomics. 13:276.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9(8):772.
- Hamilton RV, Marcus JM, Lalonde MML. 2020. The complete mitochondrial genome of the black dead leaf butterfly Doleschallia melana (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 5: 3306–3308.
- Huelsenbeck JP, Rannala B. 1997. Phylogenetic methods come of age: testing hypotheses in an evolutionary context. Science. 276(5310):227–232.
- Kodandaramaiah U, Wahlberg N. 2007. Out-of-Africa origin and dispersal-mediated diversification of the butterfly genus Junonia (Nymphalidae: Nymphalinae). J Evol Biol. 20(6):2181–2191.
- Lalonde MML, Marcus JM. 2019a. The complete mitochondrial genome of the Madagascar banded commodore butterfly Precis andremiaja (Insecta: Lepidoptera: Nymphalidae. Mitochondr DNA B Resour. 4(1):277–279.
- Lalonde MML, Marcus JM. 2019b. The complete mitochondrial genome of the Madagascar mother-of-pearl butterfly Salamis anteva (Insecta: Lepidoptera: Nymphalidae). Mitochondr DNA B Resour. 4(1):296–298.
- Lalonde MML, Marcus JM. 2020. The complete mitochondrial genome of the Malagasy clouded mother-of-pearl butterfly Protogoniamorpha ancardii duprei (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 5: 3261–3263.
- Larsen TB. 2005. Butterflies of West Africa. Stenstrup, Denmark: Apollo.
- Laslett, D, Canback, B 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24:172–175.
- Liao F, Wang L, Wu S, Li Y-P, Zhao L, Huang G-M, Niu C-J, Liu Y-Q, Li M-G. 2010. The complete mitochondrial genome of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). Int J Biol Sci. 6(2):172–186.
- Living Prairie Mitogenomics Consortium 2020. The complete mitochondrial genome of the brown pansy butterfly, Junonia stygia (Aurivillius, 1894), (Insecta: Lepidoptera: Nymphalidae). Mitochondr DNA B Resour. 5:41–43.
- Marcus JM. 2018. Our love-hate relationship with DNA barcodes, the Y2K problem, and the search for next generation barcodes. AIMS Genet. 5(1):1–23.
- McCullagh BS, Marcus JM. 2015. The complete mitochondrional genome of Lemon Pansy, Junonia lemonias (Lepidoptera: Nymphalidae: Nymphalinae. J Asia-Pacific Ent. 18(4):749–755.
- Nyafwono M, Valtonen A, Nyeko P, Roininen H. 2014. Fruit-feeding butterfly communities as indicators of forest restoration in an Afro-tropical rainforest. Biol Conserv. 174:75–83.
- Peters MJ, Marcus JM. 2017. Taxonomy as a hypothesis: testing the status of the Bermuda buckeye butterfly Junonia coenia bergi (Lepidoptera: Nymphalidae. Syst Entomol. 42(1):288–300.
- Shirôzu T, Nakanishi A. 1984. A Revision of the Genus Kallima DOUBLEDAY (Lepidoptera, Nymphalidae): I. Generic classification. Tyô to Ga (Lepidoptera Science). 34:97–110.
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 7:539.
- Swofford DL. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, Massachusetts, USA: Sinauer Associates.
- Wahlberg N, Brower AVZ, Nylin S. 2005. Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae. Biol J Linn Soc. 86(2):227–251.
- Wahlberg N, Leneveu J, Kodandaramaiah U, Peña C, Nylin S, Freitas AVL, Brower AVZ. 2009. Nymphalid butterflies diversify following near demise at the Cretaceous/tertiary boundary. Proc Biol Sci. 276(1677):4295–4302.
