Abstract
Bulleyia Schltr. is monotypic and represented only by Bulleyia yunnanensis Schltr., native to the Yunnan of China, Bhutan, and northeast India. Here, we report the complete chloroplast (cp) genome sequence and the cp genome features of B. yunnanensis. The cp genome sequence of B. yunnanensis was 159,581 bp in length and presented a typical quadripartite structure consisting of one large single-copy region (LSC, 87,563 bp), one small single-copy region (SSC, 18,714 bp), and two inverted repeat regions (IR, 26,652 bp). Besides, the cp genome encoded 132 genes, including 113 unique genes (79 protein-coding genes, 30 tRNA genes, and four rRNA genes). The phylogenetic analysis suggested that B. yunnanensis be closely related to Pholidota in tribe Arethuseae.
Bulleyia yunnanensis is a perennial epiphytic orchid with high medicinal value (Lu and Zhang Citation2015; Wang et al. Citation2015), this species is characterized by compressed pseudobulbs, pendulous racemose with 10–20 white, small and distichous flowers (Chen et al. Citation2009). According to the recent classification of Orchidaceae, Bulleyia was placed in tribe Arethuseae Lindl. of subfamily Epidendroideae (Orchidaceae), in which tribal relationships were unclear (Li et al. Citation2016). Since the plastid genome would play a key role in plant systematics and evolution (Jakobsson et al. Citation2007), the complete cp genome of B. yunnanensis was assembled and analyzed, which will facilitate future research on the evolution of orchid plants and the phylogenetic of the Bulleyia.
The leaf samples of B. yunnanensis were collected from the Wild Orchid Conservation Center of Yunnan Fengchunfang Biotechnology in Fumin County, Yunnan Province, China (25°20 ′19″ N, 102°27′26″ E). The specimen was deposited in the Herbarium of Southwest Forestry University (HSFU, Lilu 20180005). The total genomic DNA was extracted from the fresh leaf using the modified CTAB procedure of Doyle and Doyle (Citation1987) and sequenced on Illumina Hiseq 2500 platform (Illumina, San Diego, CA) in Shanghai Personal Biotechnology Co., Ltd. With the chloroplast genome of Pleione formosana Hayata (GenBank accession number NC_042197) as the reference sequence, we assembled the cp genome from the clean reads by the GetOrganelle pipe-line (Jin et al. Citation2018), then annotated the new sequences by using the Geneious Prime version 2020.0.4 (Kearse et al. Citation2012). Finally, the complete cp genome sequence was submitted to the GenBank with accession number MT610368.
The complete chloroplast genome of B. yunnanensis was 159,581 bp in length and contained two inverted repeats (IR, 26,652 bp) regions, a large single-copy region (LSC, 87,563 bp), a small single-copy region (SSC, 18,714 bp). This genome sequence encoded 132 genes, including 113 unique genes (79 protein-coding genes, 30 tRNA genes, and four rRNA genes). Among them, the GC content of LSC, SSC and IR regions reached 35.2%, 30.4% and 43.3%, respectively. Besides, GC-content was 37.4% of the overall.
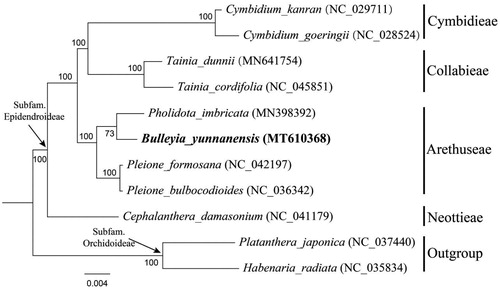
To confirm the phylogenetic position of B. yunnanensis, a maximum-likelihood (ML) tree was constructed based on 78 protein-coding genes of nine species from four related tribes in subfamily Epidendroideae as ingroup, with two species from subfamily Orchidoideae as outgroup. These four tribes were selected according to the updated classification of Orchidaceae (Chase et al. Citation2015; Li et al. Citation2016) including Cymbidieae Sw., Collabiinae Schltr., Arethuseae Lindl. and Neottieae Lindl. The phylogenetic tree was conducted using RAxML v8.1.11 (Stamatakis et al. Citation2008; Stamatakis Citation2014) as implemented on the Cyberinfrastructure for Phylogenetic Research (CIPRES) Science Gateway (http://www.phylo.org/, Miller et al. Citation2010). Other parameters used the default settings. It was revealed that B. yunnanensis was closely related to Pholidota in tribe Arethuseae of Epidendroideae ().
Figure 1. Phylogenetic position of Bulleyia yunnanensis inferred by maximum likelihood (ML) based on 78 protein-coding genes of nine species from dour related tribes in subfamily Epidendroideae as ingroup, with two species from subfamily Orchidoideae as outgroup. Sequences used in this study were downloaded from the NCBI GenBank database. The bootstrap values were shown next to the nodes.
Acknowledgments
We are grateful to Dr. Fei Zhao of Kunming Institute of Botany, Chinese Academy of Sciences, for his help in preparing this manuscript. We also thank Mr. Zhi-Feng Xu and Mrs. Xiao-Yun Wang from the Wild Orchid Conservation Center of Yunnan Fengchunfang Biotechnology Company for providing samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the finding of this study are openly availabie in GenBank of BCBI at https://www.ncbi.nlm,nih.gov under the accession no. MT610368.The associated BioProject, SRA, and Bio-Samplenumbers are PRJN664314,SAMN1625754 and SPX9148609 respectively.
Additional information
Funding
References
- Chase MW, Cameron KM, Freudenstein JV, Pridgeon AM, Salazar G, Berg CVD, Schuiteman A. 2015. An updated classification of Orchidaceae. Bot J Linn Soc. 177(2):151–174.
- Chen SC, Liu ZJ, Zhu GH, Lang KY, Ji ZH, Luo YB, Jin XH, Cribb PJ, Wood JJ, Gale SW. 2009. Orchidaceae. In: Wu ZY, Raven PH, Hong D, editors. Flora of China, Vol. 25. Beijing (China): Science Press; St. Louis (MO): Missouri Botanical Garden Press; p. 211–235.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure from small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Jakobsson M, Säll T, Lind-Halldén C, Halldén C. 2007. The evolutionary history of the common chloroplast genome of Arabidopsis thaliana and A. suecica. J Evol Biol. 20(1):104–121.
- Jin JJ, Yu WB, Yang JB, Song Y, Yi TS, Li DZ. 2018. GetOrganelle: a simple and fast pipeline for de novo assembly of a complete circular chloroplast genome using genome skimming data. bioRxiv, 256479.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649.
- Li MH, Zhang GQ, Lan SR, Liu ZJ. 2016. A molecular phylogeny of Chinese orchids. J Syst Evol. 54(4):349–362.
- Lu ZY, Zhang RG. 2015. Traditional Chinese medicine external lotion for treating anal eczema.
- Miller MA, Wayne P, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop (GCE), New Orleans, LA, 1–8.
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol. 57(5):758–771.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Wang XX, Shi HL, Sun YJ, Han JJ. 2015. Traditional Chinese medicine for treating allergic rhinitis in children.
