Abstract
The white peacock butterfly Anartia jatrophae saturata Staudinger, 1884 (Nymphalidae: Nymphalinae: Victorini), lives in the neotropics. Genome skimming with Illumina sequencing of A. jatrophae saturata allowed the assembly of a complete circular mitogenome of 15,297 bp, consisting of 81.4% AT nucleotides, 22 tRNAs, 13 protein-coding genes, two rRNAs, and a control region. Anartia jatrophae COX1 features an atypical start codon (CGA); ATP6, COX1, ND1, ND4, ND4L, ND5, and ND6 exhibit incomplete stop codons completed in the mRNA by the addition of 3′ A residues. Contrary to previous phylogenetic hypotheses, phylogenetic reconstruction places A. jatrophae as sister to nymphalid tribe Nymphalini.
The white peacock butterfly, Anartia jatrophae saturata, Staudinger, 1884, is found in open and disturbed environments as well as secondary forests throughout the Greater Antilles and Florida (Turner and Turland Citation2017; Pfeiler et al. Citation2020). Anartia jatrophae is a geographically widespread Neotropical species that is known for its territorial behavior (Lederhouse et al. Citation1992; Blum et al. Citation2003). The subspecies, A. jatrophae saturata, exhibits pronounced sexual dimorphism as well as brightly colored and faded phenotypes, which were once thought to be the result of seasonal variation, but have since been seen throughout the year (Munroe Citation1942; Turner and Turland Citation2017). Here we report the complete mitochondrial genome sequence of A. jatrophae saturata (Genbank MT712074) from specimen Anja2017.1, collected in the Dominican Republic (GPS 18.736 N, 70.163 W) in December 2017 that has been pinned, spread, and deposited in the Wallis Roughley Museum of Entomology, University of Manitoba (voucher WRME0507733).
DNA was prepared (McCullagh and Marcus Citation2015) and sequenced by Illumina NovaSeq6000 (San Diego, California) (Marcus Citation2018). The sequencing library was prepared using NEBNext Ultra II DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, Massachusetts). The mitogenome of A. jatrophae saturata was assembled by Geneious 10.1.2 from 11,195,835 paired 150 bp reads (Genbank SRA accession PRJNA65761) using a Junonia stygia (Lepidoptera: Nymphalidae) reference mitogenome (MN623383) (Living Prairie Mitogenomics Consortium Citation2020). Annotation was in reference to the J. stygia mitogenome. The A. jatrophae saturata nuclear rRNA repeat (Genbank MT742579) was also assembled and annotated using a J. stygia reference sequence.
The A. jatrophae saturata circular 15,297 bp mitogenome assembly was composed of 35,004 paired reads with nucleotide composition: 39.1% A, 11.1% C, 7.5% G, and 42.3% T. The gene composition and order in A. jatrophae saturata is identical to all known butterfly genomes (Cao et al. Citation2012; McCullagh and Marcus Citation2015). Anartia jatrophae saturata COX1 features an atypical CGA start codon as in many other insects (Liao et al. Citation2010). The mitogenome contains five protein-coding genes (COX1, ND1, ND4, ND5, ND6) with single-nucleotide (T) stop codons, and two protein-coding genes (ATP6, ND4L) with two-nucleotide (TA) stop codons completed by post-transcriptional addition of 3′ A residues. The locations and structures of tRNAs were determined using ARWEN v.1.2 (Laslett and Canback Citation2008). tRNAs have typical cloverleaf secondary structures except for trnS (AGN) where the dihydrouridine arm is replaced by a loop, while the mitochondrial rRNAs and control region are typical for Lepidoptera (McCullagh and Marcus Citation2015).
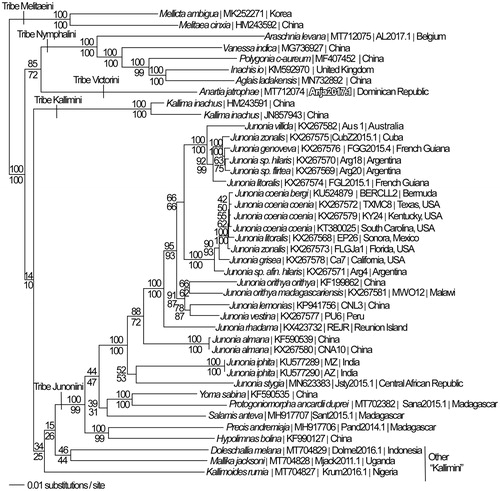
We reconstructed a phylogeny using complete mitogenomes from A. jatrophae saturata and 41 additional mitogenomes from subfamily Nymphalinae (Lalonde and Marcus Citation2019a, Citation2019b; Chen et al. Citation2020; Alexiuk et al. Citation2020a, Citation2020b; Hamilton et al. Citation2020; Lalonde and Marcus Citation2020; Payment et al. Citation2020). Mitogenome sequences were aligned in CLUSTAL Omega (Sievers et al. Citation2011) and analyzed by parsimony and maximum likelihood (model selected by jModeltest 2.1.7 (Darriba et al. Citation2012) and likelihood ratio test (Huelsenbeck and Rannala Citation1997)) in PAUP* 4.0 b/4.0d78 (Swofford Citation2002) (). Previous phylogenetic analyses based on smaller sequence data sets have placed A. jatrophae within the nymphalid tribe Victorini (Blum et al. Citation2003; Wahlberg et al. Citation2005), and have placed Victorini as sister to either tribe Junonini (Wahlberg et al. Citation2005; Kodandaramaiah and Wahlberg Citation2007) or to species that have often been assigned to tribe Kallimini (Wahlberg et al. Citation2009). Tribe Kallimini is not monophyletic in our analysis. Contrary to these earlier phylogenetic hypotheses, we have found that A. jatrophae saturata, the only representative of nymphalid tribe Victorini currently with a sequenced mitogenome, is sister to a clade of mitogenomes from tribe Nymphalini.
Figure 1. Maximum likelihood phylogeny (GTR + G model, G = 0.2330, likelihood score 117762.66543) of Anartia jatrophae saturata (tribe Victorini), 29 mitogenomes from tribe Junonini, 5 from Kallimini, 5 from Nymphalini and 2 outgroup from tribe Melitaeini in subfamily Nymphalinae based on 1 million random addition heuristic search replicates (with tree bisection and reconnection). One million maximum parsimony heuristic search replicates produced 16 trees (parsimony score 20,698 steps) which differ from one another only by the arrangement of Junonia coenia mitogenomes and one of which has an identical tree topology to the maximum likelihood tree depicted here. Numbers above each node are maximum likelihood bootstrap values and numbers below each node are maximum parsimony bootstrap values (each from 1 million random fast addition search replicates).
Acknowledgements
We thank Mackenzie Alexiuk and Rayna Hamilton for their constructive criticism on this manuscript and Genome Quebec for assistance with library preparation and sequencing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov, reference numbers MT712074, MT742579, and PRJNA657614.
Additional information
Funding
References
- Alexiuk MR, Marcus JM, Lalonde MML. 2020a. The complete mitochondrial genome and phylogenetic analysis of the European map butterfly Araschnia levana (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour.
- Alexiuk MR, Marcus JM, Lalonde MML. 2020b. The complete mitochondrial genome of the Jackson’s Leaf butterfly Mallika jacksoni (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour.
- Blum MJ, Bermingham E, Dasmahapatra K. 2003. A molecular phylogeny of the neotropical butterfly genus Anartia (Lepidoptera: Nymphalidae). Mol Phylogen Evol. 26(1):46–55.
- Cao YQ, Ma C, Chen JY, Yang DR. 2012. The complete mitochondrial genomes of two ghost moths, Thitarodes renzhiensis and Thitarodes yunnanensis: the ancestral gene arrangement in Lepidoptera. BMC Genomics. 13:276.
- Chen K, Si C, Pan Z, Hao J. 2020. The complete mitochondrial genome of Aglais ladakensis (Lepidoptera: Nymphalidae: Nymphalinae. Mitochondrial DNA B Resour. 5(1):639–641.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9(8):772
- Hamilton RV, Marcus JM, Lalonde MML. 2020. The complete mitochondrial genome of the black dead leaf Butterfly Doleschallia melana (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour.
- Huelsenbeck JP, Rannala B. 1997. Phylogenetic methods come of age: testing hypotheses in an evolutionary context. Science. 276(5310):227–232.
- Kodandaramaiah U, Wahlberg N. 2007. Out-of-Africa origin and dispersal-mediated diversification of the butterfly genus Junonia (Nymphalidae: Nymphalinae). J Evol Biol. 20(6):2181–2191.
- Lalonde MML, Marcus JM. 2019a. The complete mitochondrial genome of the Madagascar mother-of-pearl butterfly Salamis anteva (Insecta: Lepidoptera: NympChalidae). Mitochondrial DNA B Resour. 4(1):296–298.
- Lalonde MML, Marcus JM. 2019b. The complete mitochondrial genome of the Madagascar banded commodore butterfly Precis andremiaja (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 4(1):277–279.
- Lalonde MML, Marcus JM. 2020. The complete mitochondrial genome of the Malagasy clouded mother-of-pearl butterfly Protogoniamorpha ancardii duprei (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour.
- Laslett D, Canback B. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24(2):172–175.
- Lederhouse RC, Codella SG, Grossmueller DW, Maccarone AD. 1992. Host plant-based territoriality in the white peacock butterfly Anartia jatrophae (Lepidoptera: Nymphalidae). J Insect Behav. 5(6):721–728.
- Liao F, Wang L, Wu S, Li Y-P, Zhao L, Huang G-M, Niu C-J, Liu Y-Q, Li M-G. 2010. The complete mitochondrial genome of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). Int J Biol Sci. 6(2):172–186.
- Living Prairie Mitogenomics Consortium. 2020. The complete mitochondrial genome of the brown pansy butterfly, Junonia stygia (Aurivillius, 1894), (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 5:41–43.
- Marcus JM. 2018. Our love-hate relationship with DNA barcodes, the Y2K problem, and the search for next generation barcodes. AIMS Genetics. 5(1):1–23.
- McCullagh BS, Marcus JM. 2015. The complete mitochondrional genome of Lemon Pansy, Junonia lemonias (Lepidoptera: Nymphalidae: Nymphalinae). J Asia-Pacific Ent. 18(4):749–755.
- Munroe EG. 1942. The Caribbean races of Anartia jatrophae Johansson (Lepidoptera: Nymphalidae). Am Mus Novit. 1179:1–4.
- Payment JE, Marcus JM, Lalonde MML. 2020. The complete mitochondrial genome of the African leaf butterfly Kallimoides rumia (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour.
- Pfeiler E, Nazario-Yepiz NO, Hernández-Cervantes PL, Markow TA. 2020. Mitochondrial DNA barcodes provide insight into the phylogeography and subspecies controversy in the widespread Neotropical white peacock butterfly, Anartia jatrophae (Nymphalidae: Nymphalinae). Biol J Linn Soc.
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 7:539
- Swofford DL. 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland (MA): Sinauer Associates.
- Turner T, Turland V. 2017. Discovering Jamaican butterflies and their relationships around the Caribbean. Safety Habor (FL): Caribbean Wildlife Publications.
- Wahlberg N, Brower AVZ, Nylin S. 2005. Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalinae (Lepidoptera: Nymphalidae). Biol J Linn Soc. 86(2):227–251.
- Wahlberg N, Leneveu J, Kodandaramaiah U, Peña C, Nylin S, Freitas AVL, Brower AVZ. 2009. Nymphalid butterflies diversify following near demise at the Cretaceous/Tertiary boundary. Proc Biol Sci. 276(1677):4295–4302.
