Abstract
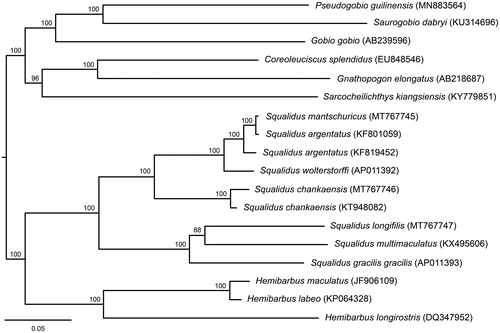
Mitochondrial genomes of Squalidus mantschuricus, S. chankaensis, and S. longifilis have been determined using Sanger sequencing (GenBank Accession No. MT767745–MT767747). The three mitochondrial genomes consist of 13 protein-coding genes, two rRNA genes, 22 tRNA genes, and one control region with the length of 16,605, 16,611, and 16,607 bp. Phylogenetic analysis of the three species showed that S. mantschuricus is nested within a fully supported terminal clade with S. argentatus, and S. chankaensis is a sister group of S. mantschuricus, S. argentatus, and S. wolterstorffi. Squalidus longifilis is positioned in a clade with S. multimaculatus and S. gracilis.
An updated classification system of the order Cypriniformes proposed by Tan and Armbruster (Citation2018) places the genus Squalidus into the family Gobionidae. Squalidus fishes are comprised about 14 species (Fricke et al. Citation2020), and they mainly live in streams and rivers of East Asia (Yue Citation1998). Squalidus longifilis is treated as valid species in a recent study (Chai Citation2020). In this study, three mitochondrial genomes were obtained using Sanger sequencing: S. mantschuricus, S. chankaensis, and S. longifilis. These data are useful for species delimitation and phylogenetic reconstruction.
Squalidus mantschuricus (voucher FDZM-SqMDH20110719-01) was sampled from Dunhua City, Jilin province, China (43.69°N, 128.60°E), S. chankaensis (FDZM-SqCJal20100719-01) from Jalaid Banner, Inner Mongolia Autonomous Region, China (46.78°N, 122.68°E), and S. longifilis (FDZM-SqLFengC20170820-01) from Fengcheng County, Liaoning province, China (40.46°N, 124.11°E). All specimens were deposited in the Zoological Museum of Fudan University (FDZM), China. Total genomic DNA was extracted from muscle tissue using a high-salt method (Miller et al. Citation1988). Thirteen pairs of primers were designed to amplify contiguous, overlapping segments using the polymerase chain reaction (PCR). The primers were as follows: Gob12sF 5′-AAGGCATGGTCCYGACCTTA-3′ and Gob16sR 5′-TTCGGTAGGTCTRTCACTTC-3′ (Primer1); Gob16sF 5′-ACCTTGTACCTTTTGCATC-3′, and GobLeuR 5′-GGGAA
GAGGAYTTGAACC-3′ (Primer2); GobND1F 5′-GCAGCCGCTATTAAGGGTT-3′ and GobND1R 5′-GGRTTCATTGATGGAGGA-3′ (Primer3); GobIleF 5′-GCCCAAG GACCACTTTGATAG-3′ and GobCOIR 5′-CCAAATACRAGATARAGGT-3′ (Primer4); GobAsnF 5′-AGCGAGCATCCATCTACTT-3′, and GobSerR 5′-GGTYATG
TGACTGGCTTGA-3′ (Primer5); GobCOIF 5′-TGAGAAGCCTTYGCCGCYAAACG′-3′ and GobATP6R 5′-AGGAATACYATYAGGGAGGC-3′ (Primer6); GobATP6F 5′-CCTTGAGAYTGACCATGAT-3′ and GobArgR 5′-CTGAGYCGAAATCAGAGG-3′ (Primer7); GobCOIIIF 5′-TGATGAGGCTCATATCTTTCTA-3′ and GobND4R 5′-TCTGTGGCRCCRAATGCTAT-3′ (Primer8); GobND4F 5′-TAGCATTTCAYCGC ACMC-3′ and GobLeuR 5′-TGGAYTTGCACCAAGAGT-3′ (Primer9); GobSerF 5′-ACTYACCRAGGAAGGACA-3′ and GobND5R 5′-TCCYCAGGCAAGYCGTTT-3′ (Primer10); GobND5F 5′-ATTGARGCCCTAAACACCTC-3′, and CGobCytbR 5′-AA GTGGAAKGCGAARAATCG-3′ (Primer11); GobND6F 5′-AAAATAGGTCATAA
TTCTTGCTCGG-3′ and GobProR 5′-GTTTAATTTAGAATTCTGGCTTTGG-3′ (Primer12); GobDloopF 5′-AAAGCATCGGTCTTGTAATC-3′, and GobDloopR 5′-CTTGGCTAGGCGTCTTGG-3′ (Primer13). Thermocycling parameters were comprised 35 thermal cycles, denaturation at 94 °C for 50 s, annealing at 52.6–55.6 °C for 60 s, and extension at 72 °C for 70 s on a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA). The PCR products were purified and directly sequenced using the PCR primers in an ABI 3730 DNA sequencer (Applied Biosystems, Foster City, CA). The assembly and annotation of mitochondrial genomes followed the methods published for Pseudorasbora elongata by Chen et al. (Citation2015). The maximum likelihood (ML) analysis was inferred using five partitions (each codon of all protein-coding genes, 12S + 16S rRNA genes, and all tRNA genes) and IQ-TREE version 1.6.2 (Nguyen et al. Citation2015) with 1000 ultrafast bootstraps (UFBoot) (Hoang et al. Citation2018). The best substitution model was estimated with ModelFinder (Kalyaanamoorthy et al. Citation2017) following the BIC criterion.
The lengths of new mitochondrial genomes are 16,605, 16,611, and 16,607 bp for S. mantschuricus, S. chankaensis, and S. longifilis, and contain the A + T base compositions of 56.0, 55.5, and 54.0%, respectively. Gene compositions, codon uses and gene arrangements are similar to previously published gobionid mitochondrial genomes (Chen and Fu Citation2019; Fu and Fu Citation2020; Yi and Fu Citation2020). For 13 protein-coding genes, start codons include ATG or GTG, and stop codons consist of TAA, TAG, TA–, or T—. Results of the inferred ML tree () show that the genus Squalidus is a monophyletic taxon. Squalidus mantschuricus is nested in a clade with S. argentatus with high bootstrap support. Squalidus chankaensis is a sister species to S. mantschuricus, S. argentatus, and S. wolterstorffi, and is fully supported. Squalidus longifilis and S. multimaculatus are positioned in a well-supported clade with S. gracilis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Three new mitochondrial genomes with accession numbers have been released in the GenBank: MT767745 (https://www.ncbi.nlm.nih.gov/nuccore/ MT767745), MT767746 (https://www.ncbi.nlm.nih.gov/nuccore/ MT767746), and MT767747 (https://www.ncbi.nlm.nih.gov/nuccore/ MT767747).
Additional information
Funding
References
- Chai J. 2020. Studies on phylogenetic relationships, historical biogeography and phylogeography of Squalidus fishes [PhD thesis]. Shanghai, China: University of Fudan.
- Chen AH, Xia R, Lei GC, Fu CZ. 2015. Complete mitochondrial genome of Pseudorasbora elongata (Cypriniformes: Cyprinidae). Mitochondrial DNA. 26(2):250–251.
- Chen Y, Fu CZ. 2019. Three complete mitochondrial genomes of freshwater fishes in the genus Abbottina (Cypriniformes: Gobionidae). Mitochondrial DNA Part B. 4(2):2179–2180.
- Fricke R, Eschmeyer WN, Van der Laan R. editors. 2020. Eschmeyer’s catalog of fishes: genera, species, references. [accessed 2020 Sep 14]. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
- Fu JW, Fu CZ. 2020. Three mitochondrial genomes of Pseudogobio fishes (Cypriniformes: Gobionidae). Mitochondrial DNA Part B. 5(3):3064–3065.
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol E. 35(2):518–522.
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. Model finder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–591.
- Miller SA, Dykes DD, Polesky HF. 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16(3):1215.
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol E. 32(1):268–274.
- Tan M, Armbruster JW. 2018. Phylogenetic classification of extant genera of fishes of the order Cypriniformes (Teleostei: Ostariophysi). Zootaxa. 4476(1):6–39.
- Yi TY, Fu CZ. 2020. Two mitochondrial genomes of freshwater gudgeons in the genus Gobio (Cypriniformes: Gobionidae). Mitochondrial DNA Part B. 5(3):3072–3073.
- Yue PQ. 1998. Fauna sinica. Osteichthyes. Cypriniformes II. Beijing, China: Science Press.