Abstract
The complete mitochondrial genome (mitogenome) of the peaceful betta (Betta imbellis) was obtained using next-generation sequencing. The sample of B. imbellis was collected from its native habitat in Southern Thailand. The mitogenome sequence was 16,897 bp in length, containing 37 genes with identical order to most teleost mitogenomes. Overall nucleotide base composition of the complete mitogenome was determined as AT bias. Phylogenetic analysis of B. imbellis showed a closer relationship with bubble-nesting fighting fish. This annotated mitogenome reference can be utilized as a bioresource for phylogenetic studies to support betta conservation programs.
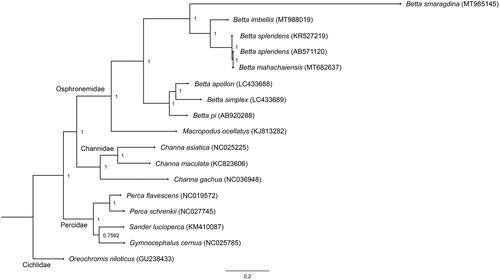
Southeast Asia (SEA) is a well-known biocultural hotspot of biodiversity and endemism encompassing a huge variety of betta fish (Betta spp.) belonging to the family Osphronemidae (Panijpan et al. Citation2014). The bubble-nesting species Betta imbellis, also known as the peaceful betta, is native to SEA and one of the most popular wild type betta fish for fishkeeping hobbyists (Kusrini et al. Citation2015). This betta fish inhabits still and sluggish waters including rice paddies, swamps, roadside ditches, streams, and ponds. Artificial selection activities for ornamental and aquarium trade purposes have resulted in inbreeding and outbreeding depression with an adverse effect on the genetic integrity of wild populations (Beer et al. Citation2019). Here, a complete mitochondrial genome (mitogenome) of B. imbellis collected from Nakhon Si Thammarat, Thailand (8.4325° N, 99.9599° E), was assembled, annotated, analyzed, and stored in the Thailand Natural History Museum (no. THM21221). Whole genomic DNA was extracted in accordance with the standard salting-out protocol (Supikamolseni et al. Citation2015), and next-generation sequencing was performed using an Illumina HiSeq platform at Vishuo Biomedical (Thailand) Ltd. (Bangkok, Thailand). Quality of Illumina reads was evaluated with FastQC software and the raw reads were trimmed to discard adapters using Trimmomatic (Bolger et al. Citation2014). The trimmed reads were subjected to alignments to isolate all mitogenome sequences by mapping whole genome Illumina reads against the complete mitogenome of B. splendens (AB571120), using bwa default parameters (Li and Durbin Citation2009). The mapped alignment was processed using Samtools (Li et al. Citation2009), and aligned reads were extracted using Bedtools (Quinlan and Hall Citation2010). Aligned reads with the mitogenome were then de novo assembled using Velvet (Velvet_1.1.07; kmer = 123) (Zerbino and Birney Citation2008). A total of 395,074 individual reads gave a mean coverage of more than 330X for the generated contigs. We then assembled a consensus scaffold of the complete mitogenome using the reference-based assembly approach in Geneious Prime (https://www.geneious.com/prime/), by mapping the Velvet contigs against the reference mitogenome of B. splendens. The assembled mitogenome was annotated in the MITOS WebServer (Bernt et al. Citation2013). Complete mitogenome sequences consisted of 16,897 bp for B. imbellis (GenBank Accession number: MT988019, SRA: SRR12614920, BioProject: PRJNA662470), containing 37 genes and a control region (CR). Gene arrangement patterns were identical to those of teleosts (Miya et al. Citation2013). Overall AT content values for the mitogenome were 61.9%. Average nucleotide diversity among all Betta mitogenomes was determined at 24.56 ± 6.04%. Four conserved sequence blocks (CSB-D, CSB1, CSB2, and CSB3) in the CR of teleost mitogenomes were also present in B. imbellis (Lee and Kocher Citation1995; Prakhongcheep et al. Citation2018; Ponjarat et al. Citation2019; Singchat et al. Citation2020). Diverse numbers of tandem repeats were observed among Betta species (Song et al. Citation2016; Prakhongcheep et al. Citation2018; Ponjarat et al. Citation2019; Singchat et al. Citation2020), suggesting that the CR had large variation in different fighting fish species. A phylogenetic tree was constructed based on 12 concatenated protein-coding genes without ND6 of 17 teleosts, using Bayesian inference with MrBayes version 3.2.6 (Huelsenbeck and Ronquist Citation2001). The group comprising B. splendens, B. mahachaiensis, B. smaragdina, and B. imbellis formed a monophyletic clade as bubble-nesting fighting fish, consistent with Ruber et al. (Citation2004), Sriwattanarothai et al. (Citation2010), and Singchat et al. (Citation2020) (). These complete mitogenomes comprise a reference annotated genome, and provide valuable information at the molecular level that can be utilized to sustain betta bioresources to improve conservation programs and commercial breeding management.
Figure 1. Phylogenetic relationships among 12 concatenated mitochondrial protein-coding genes, without ND6 sequences of 17 mitochondrial genomes including Oreochromis niloticus as the outgroup, using Bayesian inference analysis. The complete mitochondrial genome sequence was downloaded from GenBank. Accession numbers are indicated in parentheses after the scientific names of each species. Support values at each node are Bayesian posterior probabilities, while branch lengths represent the number of nucleotide substitutions per site.
Acknowledgements
The authors would like to thank Sunchai Makchai (Thailand Natural History Museum) for advice on sample preparation. We are also indebted to Vishuo Biomedical (Thailand) Ltd. for excellent service collaboration. The Center for Agricultural Biotechnology (CAB) at Kasetsart University Kamphaeng Saen Campus provided support with server analysis services, and the Faculty of Science and the Faculty of Forestry at Kasetsart University supported the research facilities. The National Biobank of Thailand (NBT) under the National Science and Technology Development Agency (NSTDA), Thailand supported this study through the use of a high-performance computer. We also thank the Plakad Association, Thailand for providing information concerning Betta spp. in Thailand.
Disclosure statement
The authors report no conflicts of interest and are entirely responsible for the contents of this article. Animal care and all experimental procedures were approved by the Animal Experiment Committee, Kasetsart University, Thailand (approval no. ACKU63-SCI-007) and conducted in accordance with the Regulations on Animal Experiments at Kasetsart University.
Data availability statement
Data supporting the findings of this study are openly available in SRA and GenBank of NCBI at https://www.ncbi.nlm.nih.gov. The isolated mitogenome reads were deposited at the NCBI SRA database (accession ID: SRR12614920), and assembled mitogenome sequences are available in GenBank (accession ID: MT988019) under the BioProject: PRJNA662470.
Additional information
Funding
References
- Beer SD, Cornett S, Austerman P, Trometer B, Hoffman T, Bartron ML. 2019. Genetic diversity, admixture, and hatchery influence in Brook Trout (Salvelinus fontinalis) throughout western New York State. Ecol Evol. 9(13):7455–7479.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina Sequence Data. Bioinformatics. 30(15):2114–2120.
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 17(8):754–755.
- Kusrini E, Rahmawati R, Murniasih S, Kusumah RV, Prasetio AB. 2015. Growth and colour performance of the crossbreed marble strain Betta splendens and Betta imbellis. Indonesian Aquacult J. 10(2):101–112.
- Lee WJ, Kocher TD. 1995. Complete sequence of a sea lamprey (Petromyzon marinus) mitochondrial genome: early establishment of the vertebrate genome organization. Genetics. 139(2):873–887.
- Li H, Durbin H. 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 25(14):1754–1760.
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R; 1000 Genome Project Data Processing Subgroup. 2009. The sequence alignment/Map format and SAMtools. Bioinformatics. 25(16):2078–2079.
- Miya M, Friedman M, Satoh TP, Takeshima H, Sado T, Iwasaki W, Yamanoue Y, Nakatani M, Nakatani K, Inoue JG, et al. 2013. Evolutionary origin of the Scombridae (Tunas and Mackerels): members of a paleogene adaptive radiation with 14 other pelagic fish families. PLoS One. 8(9):e73535.
- Panijpan B, Kowasupat C, Laosinchai P, Ruenwongsa P, Phongdara A, Senapin S, Wanna W, Phiwsaiya K, Kühne J, Fasquel F. 2014. Southeast Asian mouth-brooding Betta fighting fish (Teleostei: Perciformes) species and their phylogenetic relationships based on mitochondrial COI and nuclear ITS1 DNA sequences and analyses. Meta Gene. 2:862–879.
- Ponjarat J, Areesirisuk P, Prakhongcheep O, Dokkaew S, Sillapaprayoon S, Muangmai N, Peyachoknagul S, Srikulnath K. 2019. Complete mitochondrial genome of two mouthbrooding fighting fishes, Betta apollon and B. simplex (Teleostei: Osphronemidae). Mitochondrial DNA B Resour. 4(1):672–674.
- Prakhongcheep O, Muangmai N, Peyachoknagul S, Srikulnath K. 2018. Complete mitochondrial genome of mouthbrooding fighting fish (Betta pi) compared with bubble nesting fighting fish (B. splendens). Mitochondrial DNA B Resour. 3(1):6–8.
- Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 26(6):841–842.
- Ruber L, Britz R, Tan HH, Ng PKL, Zardoya R. 2004. Evolution of mouthbrooding and life-history correlates in the fighting fish genus Betta. Evolution. 58(4):799–813.
- Singchat W, Ahmad SF, Laopichienpong N, Suntronpong A, Pongsanarm T, Panthum T, Ariyaraphong N, Subpayakom N, Dokkaew S, Muangmai N, et al. 2020. Complete mitochondrial genome of Mahachai betta, Betta mahachaiensis (Teleostei: Osphronemidae). Mitochondrial DNA B Resour. 5(3):3077–3079.
- Song YN, Xiao GB, Li JT. 2016. Complete mitochondrial genome of the Siamese fighting fish (Betta splendens). Mitochondrial DNA A DNA Mapp Seq Anal. 27(6):4580–4581.
- Sriwattanarothai N, Steinke D, Ruenwongsa P, Hanner R, Panijpan B. 2010. Molecular and morphological evidence supports the species status of the Mahachai fighter Betta sp. Mahachai and reveals new species of Betta from Thailand. J Fish Biol. 77(2):414–424.
- Supikamolseni A, Ngaoburanawit N, Sumontha M, Chanhome L, Suntrarachun S, Peyachoknagul S, Srikulnath K. 2015. Molecular barcoding of venomous snakes and species-specific multiplex PCR assay to identify snake groups for which antivenom is available in Thailand. Genet Mol Res. 14(4):13981–13997.
- Zerbino D, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18(5):821–829.
