Abstract
Phyllostachys angusta McClure is a precious wood-use bamboo resource, with almost straight stem. The complete chloroplast genome of the P. angusta McClure was assembled for the first time from Illumina pair-end sequencing data in this work. The total genome size of P. angusta McClure was 139,678 bp in length, containing a large single-copy (LSC) region of 83,212 bp, a small single-copy (SSC) region of 12,870 bp, and a pair of inverted repeat (IR) regions of 21,798 bp. The overall GC content of the genome was 38.89%, and the corresponding values of the LSC, SSC, and IR regions were 36.97, 33.17, and 44.22%, respectively. A total of 131 genes were annotated, including 85 protein-coding genes, 36 tRNA genes, and 8 rRNA genes. Phylogenetic analysis results strongly supported that P. angusta McClure was closely related to P. reticulate.
The culm wall of P. angusta McClure, belonging to Phyllostachys, is particularly straight, which is mainly cultivated in Zhejiang Provinces of China. The chloroplast genomes of Phyllostachys genus have been reported as Phyllostachys edulis cultivar pachyloen, Phyllostachys edulis, Phyllostachys nigra var. henonis, Phyllostachys reticulata, and Phyllostachys sulphurea (Zhang et al. Citation2011; Wu and Ge Citation2012; Gao and Gao Citation2016; Huang et al. Citation2020). In the present study, we reported the complete cp genome sequence of Phyllostachys angusta McClure based on Illumina pair-end data for the first time. We also explored its phylogenetic relationship with other plant species, which would help our better understanding of the evolution of Phyllostachys cp genome.
The fresh leaves of Phyllostachys angusta McClure were collected from the experimental bamboo forest (119.236° E, 30.698° N, 36.9 m above sea level) in Shimen Town, Xuancheng County, Anhui Province, China. The voucher specimens have been deposited in East China Inventory and Planning Institute of National Forestry and Grassland Administration (20200813). Total genome DNA was extracted with the Qiagen plant genomic DNA prep kit (Sangon Biotech, Shanghai, China), which were sequence using the Illumina HiSeq 2500 platform. The library with insert size of 300 bp fragments was constructed and sequenced using the Illumina HiSeq platform in Novogene (Nanjing, China). The raw data were used to assemble the complete cp genome using Getorganelle software (Jin et al. Citation2018) with Phyllostachys edulis as the reference. Genome annotation was performed with the program Geneious R8 (Biomatters Ltd, Auckland, New Zealand) by comparing the sequences with the cp genome of Phyllostachys edulis, coupled with manual. The tRNA genes were further confirmed through online tRNAscan-SE web servers (Schattner et al. Citation2005). A gene map of the annotated P. heteroclada f. solida cp genome was drawn by OGdraw online (Lohse et al. Citation2013). Furthermore, the cp genome data of Phyllostachys angusta McClure was available in the NCBI under accession number of MW027348 (https://www.ncbi.nlm.nih.gov/nuccore/MW027348).
The cp genome of Phyllostachys angusta McClure was a quadripartite circular with 139,678 bp, which comprised of a large-single copy (LSC) region of 83,212 bp and a small single copy (SSC) region of 12,870 bp, separated by two inverted repeat (IR) regions of 21,798 bp, respectively. The GC content of the total genome was 36.12%, whereas the IR region had a higher GC content (44.22%) than LSC (36.97%) and SSC (33.17%). The cp genome encoded 131 genes, including 85 protein-coding genes, 36 tRNA genes, and 8 rRNA genes.
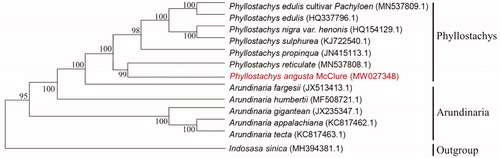
In order to study the relationship between Phyllostachys angusta McClure and other Phyllostachys plants, the cp genome data of six species of Phyllostachys (Phyllostachys edulis var. Pachyloen, Phyllostachys edulis, Phyllostachys nigra var. henonis, Phyllostachys sulphurea, Phyllostachys propinqua and Phyllostachys reticulate), five species of Arundinaria (Arundinaria fargesii, Arundinaria humbertii, Arundinaria gigantean, Arundinaria appalachiana and Arundinaria tecta) have been published in the NCBI gene library were used to align by MAFFT v7.313 (Katoh and Standley Citation2013) and construct phylogenetic trees (). Phylogenetic analysis results strongly supported that Phyllostachys angusta McClure was closely related to Phyllostachys reticulate.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession no. MW027348. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA642983, SRS6922745, and SAMN15402429, respectively.
Additional information
Funding
References
- Gao J, Gao L-Z. 2016. The complete chloroplast genome sequence of the Phyllostachys sulphurea (Poaceae: Bambusoideae). Mitochondrial DNA Part A. 27(2):983–985.
- Huang N-J, Li J-P, Yang G-Y, Yu F. 2020. Two plastomes of Phyllostachys and reconstruction of phylogenic relationship amongst selected Phyllostachys species using genome skimming. Mitochondrial DNA Part B. 5(1):69–70.
- Jin J-J, Yu W-B, Yang J-B, Song Y, Yi T-S, Li D-Z. 2018. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. BioRxiv. 256479. http://doi.org/10.1101/256479.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Lohse M, Drechsel O, Kahlau S, Bock R. 2013. Organellar GenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 41:575–581.
- Schattner P, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33:686–689.
- Wu Z-Q, Ge S. 2012. The phylogeny of the BEP clade in grasses revisited: evidence from the whole-genome sequences of chloroplasts. Mol Phylogenet Evol. 62(1):573–578.
- Zhang Y-J, Ma P-F, Li D-Z. 2011. High-throughput sequencing of six bamboo chloroplast genomes: phylogenetic implications for temperate woody bamboos (Poaceae: Bambusoideae). PLOS One. 6(5):e20596.