Abstract
The complete mitochondrial genome was determined for the whitefly Aleyrodes shizuokensis (Hemiptera: Aleyrodidae), the first record from Chinese mainland. The mitochondrial genome is 16,687 bp in length and contains 13 protein-coding genes (PCGs), 22 transfer RNAs, and two ribosomal RNAs. The overall base composition is 33.8% A, 47.0% T, 12.2% G, and 7.0% C. All PCGs start with ATN codon. COX1 ends with a T, and the other 12 PCGs use TAA or TAG as the stop codon. Gene arrangement of the 13 PCGs is identical to that of the giant whitefly Aleurodicus dugesii and greenhouse whitefly Trialeurodes vaporariorum. The resultant Bayesian inference and maximum-likelihood trees based on the sequence data of 13 PCGs support its close relationship with sugarcane whitefly Neomaskellia andropogonis.
The whitefly Aleyrodes shizuokensis Kuwana 1911 (Hemiptera: Aleyrodidae) was recorded from Japan, Hawaii, India and Taiwan (Takahashi Citation1935; Paulson and Kumashiro Citation1985; David and Jesudasan Citation1989). In this work, A. shizuokensis was first found in Chinese mainland. In insect taxonomy, morphological characteristics and DNA barcodes are used for species identification and phylogenetic analysis. Chen et al. (Citation2007) described the puparium and adults of A. shizuokensis, providing sufficient morphological characteristics of this species. Whereas, only two DNA barcodes of A. shizuokensis are deposited in GenBank nucleotide sequence database, i.e. 16S ribosomal RNA (GQ867759) and cytochrome c oxidase subunit I (GQ867730). Mitochondrial genomes have been broadly used in phylogenetic analysis (Ma et al. Citation2012) and are powerful means for inferring ancient evolutionary relationships (Boore Citation1999). So far, the A. shizuokensis mitochondrial genome and DNA barcode-based phylogenetic analysis have not been reported.
This article reports the complete mitochondrial genome of the whitefly A. shizuokensis. The whiteflies were collected from Oxalis corniculata at Hangzhou, China (30°18′32″N, 120°5′49″E) on 6 July 2020, and deposited at the Institute of Insect Sciences, Zhejiang University, Hangzhou, China. Total genomic DNA was extracted from a female adult using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) and next-generation sequencing was performed at Illumina HiSeq 4000 platform (2 × 150 bp). Raw reads were filtered using Trimmomatic (Bolger et al. Citation2014) and clean reads were assembled using SPAdes (Bankevich et al. Citation2012). The assembled mitochondrial genome sequence was annotated with MITOS (Bernt et al. Citation2013) and tRNAscan-SE (Lowe and Eddy Citation1997). Some annotations were corrected manually.
The complete mitochondrial genome of A. shizuokensis is 16,687 bp in length (GenBank accession number: MT880225), and contains 13 protein-coding genes (PCGs), 22 tRNAs, and two rRNAs. The overall base composition is 33.8% A, 47.0% T, 12.2% G, and 7.0% C, with an A + T bias of 80.8%. All PCGs use ATN as the start codon. COX1 ends with a single T, and the other 12 PCGs use TAA or TAG as the stop codon. Gene arrangement of the 13 PCGs is identical to that of the giant whitefly Aleurodicus dugesii (AY521251) and greenhouse whitefly Trialeurodes vaporariorum (AY521265).
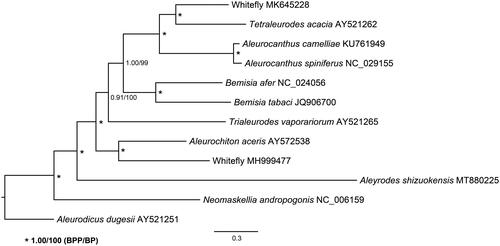
The phylogeny of available whitefly species and A. shizuokensis was analyzed based on nucleotide sequences of the 13 PCGs. The sequences were aligned using ClustalW in software MEGA (Kumar et al. Citation2016), followed by eliminating poorly aligned positions and divergent regions using Gblocks (Talavera and Castresana Citation2007). The phylogenetic relationships were reconstructed using the Bayesian inference and maximum-likelihood methods through MrBayes (Ronquist et al. Citation2012) and RAxML (Stamatakis Citation2006). The topology of the phylogenetic trees is consistent with that described in Lei et al. (Citation2019) and A. shizuokensis is close related to sugarcane whitefly Neomaskellia andropogonis ().
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov. The complete mitochondrial genome of Aleyrodes shizuokensis for this study has been deposited in GenBank with accession number MT880225. The associated BioProject, BioSample, and SRA numbers are PRJNA681367, SAMN16951332, and SRR13162646.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477.
- Bernt M, Donath A, Jühling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120.
- Boore JL. 1999. Animal mitochondrial genomes. Nucleic Acids Res. 27(8):1767–1780.
- Chen CH, Dubey AK, Ko CC. 2007. Comparative morphological studies on two species of Aleyrodes (Hemiptera: Aleyrodidae). Pan-Pac Entomol. 83(3):244–254.
- David BV, Jesudasan RWA. 1989. Redescription of the whitefly Aleyrodes shizuokensis Kuwana (Aleyrodidae: Homoptera). J Bombay Nat Hist Soc. 86:260–261.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874.
- Lei T, Liu SS, Liu YQ. 2019. The complete mitochondrial genome of a whitefly (Hemiptera: Aleyrodidae) infesting Glycine max. Mitochondrial DNA B. 4(1):2011–2012.
- Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25(5):955–964.
- Ma C, Yang PC, Jiang F, Chapuis MP, Shali Y, Sword GA, Kang L. 2012. Mitochondrial genomes reveal the global phylogeography and dispersal routes of the migratory locust. Mol Ecol. 21(17):4344–4358.
- Paulson GS, Kumashiro BR. 1985. Hawaiian Aleyrodidae. Proc Hawai Entomol Soc. 25:103–124.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22(21):2688–2690.
- Takahashi R. 1935. Notes on the Aleyrodidae of Japan (Homoptera) III. Kontyu. 9:279–283.
- Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 56(4):564–577.