Abstract
Diachrysia nadeja is a polyphagous herbivorous moth within the family Noctuidae. In this study, we sequenced and analyzed the complete mitochondrial genome (mitogenome) of D. nadeja. This mitogenome was 15,242 bp long and encoded 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs) and 2 ribosomal RNA unit genes (rRNAs). Gene order was conserved and identical to most other previously sequenced Noctuidae. Except for cox1 started with CGA, all other PCGs started with the standard ATN codons. Most of the PCGs terminated with the stop codon TAA, whereas cox1, cox2, and nad4 end with the incomplete codon T−. The whole mitogenome exhibited heavy AT nucleotide bias (80.5%). Phylogenetic analysis showed that D. nadeja got together with three Ctenoplusia species (C. agnata, C. limbirena, and C. albostriata) with high support value, indicating Diachrysia had a closer relationship with Ctenoplusia within Noctuidae.
Plusiinae (Lepidoptera: Noctuidae) is a main large subfamily of worldwide distribution, with more than 500 species worldwide, and they are spread from the tropics to the arctic (Ronkay et al. Citation2008). Species of Plusiinae can be easily distinguished from other subfamilies of Noctuidae by the following characters: golden spots or silvery stigma on the forewing, lashed eyes, and large scale tufts on the thorax, and the larvae bearing two pairs of prolegs on abdominal segments V and VI (Ronkay et al. Citation2008; Behounek et al. Citation2010). Diachrysia nadeja (Oberthür, 1880), one of the species in Plusiinae, has recorded in China, Euro-Siberian and Japan (Goater et al. Citation2003). Diachrysia nadeja is a polyphagous herbivorous moth, with the larvae feed on plants of Chenopodiaceae, Polygonaceae, Urticaceae, Fabaceae, Rubiaceae, Plantaginaceae, Lamiaceae, and Asteraceae (Leley Citation2016).
Specimens of D. nadeja were collected from Dalian City, Liaoning Province, China (38°90′N, 121°45′E, August 2019) and were stored in Entomological Museum of Anyang Institute of Technology (Accession number AIT-E-DIA02). Total genomic DNA was extracted from tissues using DNeasy DNA Extraction kit (Qiagen, Hilden, Germany). The mitogenome sequence of D. nadeja was generated using Illumina HiSeq 2500 Sequencing System (Illumina, San Diego, CA, USA). In total, 5.6 G raw reads were obtained, quality-trimmed, and assembled using MITObim v 1.7 (Hahn et al. Citation2013). By comparison with the homologous sequences of other Noctuidae species from GenBank, the mitogenome of D. nadeja was annotated using software GENEIOUS R8 (Biomatters Ltd., Auckland, New Zealand).
The complete mitogenome of D. nadeja is 15,242 bp in length (GenBank accession no. MT916722), and containing the typical set of 13 protein-coding, 2 rRNA, and 22 tRNA genes, and one non-coding AT-rich region. Gene order was conserved and identical to most other previously sequenced Noctuidae (Timmermans et al. Citation2014; Li et al. Citation2015; Yao et al. Citation2018; Huang et al. Citation2019). The overall base composition of the mitogenome was estimated to be A 39.1%, T 41.4%, C 11.6% and G 7.9%, with a high A + T content of 80.5%. Except for cox1 started with CGA, all other PCGs started with the standard ATN codons (seven ATG, two ATT, two ATA and one ATC). Most of the PCGs terminated with the stop codon TAA, whereas cox1, cox2, and nad4 end with the incomplete codon T−. The 22 tRNA genes vary from 63 bp (trnR) to 71 bp (trnK). Two rRNA genes (rrnL and rrnS) locate at trnL1/trnV and trnV/control region, respectively. The lengths of rrnL and rrnS in M. calida are 1346 and 785 bp, with the AT contents of 84.4 and 85.3%, respectively.
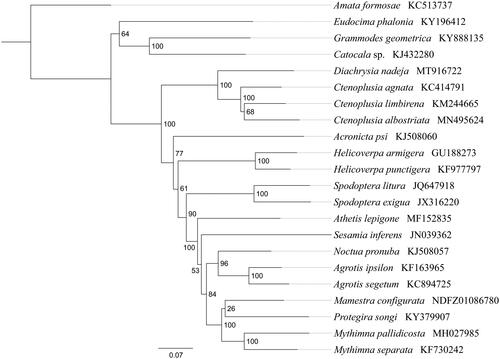
Phylogenetic analysis was performed based on the nucleotide sequences of 13 PCGs from 22 Lepidoptera species. Phylogenetic tree was constructed through raxmlGUI 1.5 (Silvestro and Michalak Citation2012) . Results showed that the new sequenced species D. nadeja got together with three Ctenoplusia species (C. agnata, C. limbirena, and C. albostriata) with high support value (BS = 100), indicating Diachrysia has a closer relationship with Ctenoplusia within Noctuidae. In conclusion, the mitogenome of D. nadeja is sequenced in this study and can provide essential DNA molecular data for further phylogenetic and evolutionary analysis of Noctuidae.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in NCBI (National Center for Biotechnology Information) at https://www.ncbi.nlm.nih.gov/, reference number MT916722.
Additional information
Funding
References
- Behounek G, Ronkay L, Ronkay G. 2010. The witt catalogue. A taxonomic atlas of the Eurasian and North African Noctuoidea Volume 4, Plusiinae II. Heterocera Press, p. 280.
- Goater B, Ronkay L, Fibiger M. 2003. Catocalinae & Plusiinae. Noctuidae Europaeae, Volume 10. Entomological Press; p. 452
- Hahn C, Bachmann L, Chevreux B. 2013. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads—a baiting and iterative mapping approach. Nucleic Acids Res. 41(13):e129–e129.
- Huang Y, Liu Y, Zhu X-Y, Xin Z-Z, Zhang H-B, Zhang D-Z, Wang J-L, Tang B-P, Zhou C-L, Liu Q-N, et al. 2019. Comparative mitochondrial genome analysis of Grammodes geometrica and other noctuid insects reveals conserved mitochondrial genome organization and phylogeny. Int J Biol Macromol. 125:1257–1265.
- Leley. 2016. Annotated catalogue of the insects of Russian Far East. Volume II. Lepidoptera. Cat Ins Russian Far East. 2:1–812.
- Li F, Wang W, Zhang H, Shen W, Xu X, Chen J, Meng Z. 2015. Complete mitochondrial genome of the oriental armyworm Mythimna separata (Walker) (Lepidoptera: Noctuidae). Mitochondr DNA. 26(6):881–882.
- Ronkay L, Ronkay G, Behounek G. 2008. The witt catalogue. A taxonomic atlas of the Eurasian and North African Noctuoidea Volume I, Plusiinae I. Heterocera Press, p. 348.
- Silvestro D, Michalak I. 2012. RaxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 12(4):335–337.
- Timmermans MJ, Lees DC, Simonsen TJ. 2014. Towards a mitogenomic phylogeny of Lepidoptera. Mol Phylogenet Evol. 79:169–178.
- Yao S, Wang ZH, Zhu XY, Hao JS. 2018. The complete mitochondrial genome of the Sideridis albicosta (Lepidoptera: Noctuoidea: Noctuidae). Mitochondr DNA B. 3(2):474–475.