Abstract
Angelica keiskei (Miq.) Koidz. is a perennial herbaceous plant belonging to the genus Angelica, Umbelliferae. As a plant with dual-purpose as food and medicine, it has the potential for the future development of high-value functional products. The complete chloroplast genome has a total size of 147,007 bp, consisting of two inverted repeats (IR, 18,508 bp, each), and separated by a large single-copy region (LSC, 92,415 bp) and a small single-copy region (SSC, 17,576 bp). Further annotation revealed the chloroplast genome contains 128 genes, including 84 protein-coding genes (80 PCG species), 36 tRNA genes (30 tRNA species), and 8 rRNA genes (4 rRNA species). A total of 83 simple sequence repeats (SSRs) were identified in the chloroplast genome. This chloroplast genome resource will be useful for the study of the evolution and genetic diversity of Angelica keiskei in the future.
Angelica keiskei (Miq.) Koidz. is a large perennial herb plant belonging to the genus Angelica, Umbelliferae, and mainly distributed in Central and Western China. Most plants in this genus are frequently used as folk medicine. The roots of A. keiskei are used in traditional Chinese medicine for replenishing blood, treating abnormal menstruation, and other diseases associated with women’s reproductive health (Chen et al. Citation2013). Angelica keiskei could help to prevent thrombotic diseases and has become popular as herbal medicine, dietary supplement, and health food in Asian countries (Ohkura et al. Citation2018). In this study, to obtain new insights into the phylogeny of A. keiskei, we sequenced, assembled, and annotated the accurate chloroplast genome.
The A. keiskei whole herb specimen was collected from Huangpi Medicinal Herb Garden, Wuhan, Hubei province of China (WH2019120508946, 114°18′58′′E, 30°34′51′′N). The voucher specimens were deposited at the Herbarium of South-Central University for Nationalities (HSN). The complete genomic DNA was extracted using CTAB method (Doyle and Doyle Citation1987) and sequenced using the Illumina NovaSeq platform at Majorbio Company (Shanghai, China). A total of 2.8 Gb raw reads were generated and low-quality sequences were filtered out. The trimmed reads were assembled using GetOrganelle (Jin et al. Citation2020). The assembled genome was annotated using CPGAVAS2 (Shi et al. Citation2019) and PGA (Qu et al. Citation2019). The complete chloroplast genome was 147,007 bp (MW125613) and composed of two inverted repeats (IRs) of 18,508 bp each, which divide a large single-copy (LSC) region of 92,415 bp and a small single-copy (SSC) region of 17,576 bp, the average GC content was 37.51%. The chloroplast genomes encoded 128 functional genes, including 84 protein-coding genes (80 PCG species), 36 tRNA genes (30 tRNA species), and 8 rRNA genes (4 rRNA species). A total of 83 SSR markers ranging from mononucleotide to hexa-nucleotide repeat motif were identified in A. keiskei chloroplast genome.
In order to explore the phylogenetic relationship of A. keiskei within Leguminosae, the complete chloroplast genomes of 32 species from Leguminosae were obtained from the GenBank database, with the Brassaiopsis hainla and Fatsia japonica as the outgroups, the phylogenetic trees were built from the whole protein-coding gene matrix by maximum-likelihood (ML) and Bayesian inference (BI) (). The ML tree was generated using IQ-TREE (Nguyen et al. Citation2015) based on the best model of TVM + F+R2 and 1000 bootstrap replicates, and BI analysis was performed in MrBayes-3.2.7 (Ronquist et al. Citation2012). This result showed that the analyzed A. keiskei were closer to the species of A. polymorpha.
Figure 1. Phylogenetic tree reconstructed by maximum-likelihood (ML) and Bayesian inference (BI) analysis basedon the whole chloroplast protein-coding genes of these 35 species.
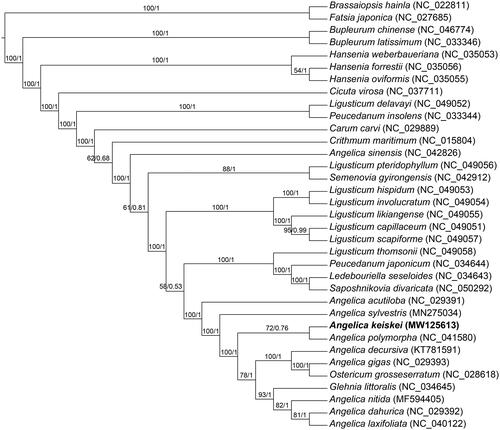
This information will be useful for the study of the evolution and genetic diversity of A. keiskei in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at [https://www.ncbi.nlm.nih.gov] (https://www.ncbi.nlm.nih.gov/) under the accession no. MW125613. The associated ‘BioProject,’ ‘SRA,’ and ‘Bio-Sample’ numbers are PRJNA670261, SRR12853312, and SAMN16491009 respectively.
Additional information
Funding
References
- Chen X-P, Li W, Xiao X-F, Zhang L-L, Liu C-X. 2013. Phytochemical and pharmacological studies on Radix Angelica sinensis. Chin J Nat Med. 11(6):577–587.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure from small quantities of fresh leaf tissues. Phytochem Bull. 19:11–15.
- Jin J-J, Yu W-B, Yang J-B, Song Y, dePamphilis CW, Yi T-S, Li D-Z. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241.
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Ohkura N, Atsumi G, Ohnishi K, Baba K, Taniguchi M. 2018. Possible antithrombotic effects of Angelica keiskei (Ashitaba). Pharmazie. 73(6):315–317.
- Qu X-J, Moore MJ, Li D-Z, Yi T-S. 2019. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods.
- Ronquist F, Teslenko M, Van Der Mark P, Ayres D L, Darling A, Höhna S, Larget B, Liu L, Suchard M A, Huelsenbeck J P. 2012. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology. 61(3):539–542. doi:10.1093/sysbio/sys029.
- Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, Liu C. 2019. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 47(W1):W65–W73.
