Abstract
The Hainan gibbon (Nomascus hainanus) is endemic to China and is the world’s rarest ape. The remaining wild population totals only 33 individuals. In the current study, we sequenced the Mitochondrial DNA control region of 12 wild Hainan gibbons representing three social groups of the five remaining groups. By conducting population genetic analyses, we found that the proportion of four nucleotides (T, C, A and G) were 29.0%, 27.2%, 31.9% and 11.9%, respectively. Hypervariable segments of the mtDNA D-loop region (1005 bp in length), indicated five variable sites (a point mutation), with only two haplotypes present among the 12 samples. We observed that the genetic diversity of Hainan gibbons is lower than that reported in any other wild primate population, and that the two haplotypes detected, represent two ancestral lineages. These findings have important implications for proposing effective conservation strategies to protect this Critically Endangered ape species.
Introduction
The Hainan gibbon (Nomascus hainanus, Hylobatidae, Primates) (Thomas Citation1892) is the world’s rarest ape, with a remaining wild population of some 33 individuals. Although once widespread across Hainan, China, the last remining population is confined to a forested area of 16 km2 in the Bawnagling National Nature Reserve. Recent estimates indicate the existence of five remaining social groups (A, B, C, D, E) (Unpublished data from 2020 monitoring results). Hainan gibbons experienced a rapid population decline in the mid 20th century, as 99.9% of their habitat was lost due to the conversion of tropical forests for purposes of industrial agriculture. In the 1970s, the Hainan gibbon population totaled only 7–8 individuals (Liu et al. Citation1984; Zhou et al. Citation2005; Deng et al. Citation2015). And, although the population has increased fourfold over the past 40 years, this species continues to face an impending extinction crisis.
Population genetics offer an important tool to better understand and model the evolutionary history, population dynamics, and patterns of gene flow of threatened species. This information is essential in developing effective strategies to manage and protect wild animal populations. For example, studies have shown that over the past few centuries, orangutans (Pongo pygmaeus) inhabiting northeastern Borneo have experienced a genetic bottleneck associated with a population decline of some 95%. This has occurred in response to anthropogenically induced habitat fragmentation, deforestation, and hunting (Goossens et al. Citation2006). Similarly, the Mexican howler monkey (Alouatta palliata mexicana), an Endangered primate subspecies is currently distributed in only four forest fragments in the state of Veracruz, Mexico. Genetic testing revealed that haplotype diversity and nucleotide diversity (h = 0.486; π = 0.0007) are extremely low compared with other Neotropical primates (Jacob et al. Citation2014). Finally, in northwestern Madagascar, populations of the golden-brown mouse lemur (Microcebus ravelobensis) have been severely reduced, resulting in a dramatic decrease in genetic diversity (Guschanski et al. Citation2007). Although the set of anthropogenic factors that promote wildlife population decline and biodiversity loss are well understood (Estrada et al. Citation2018; Estrada et al. Citation2019; Estrada et al. Citation2020), the specific effects of population decline on genetic diversity and extinction risk in individual taxa require continued investigation.
In the current study we examine levels of genetic diversity in the mitochondrial DNA (mtDNA) control region of the Critically Endangered Hainan gibbon. Previous research on the genetics of this species have focused principally on their phylogenetic position within the gibbon radiation (Su et al. Citation1995; Zhang Citation1995; Thinh et al. Citation2010a,b). To date, there has been only one study of the genetic diversity of the mitochondrial D-loop region of the Hainan gibbon (Li et al. Citation2010). However, given that the genetic data used in that study came from a single family group and focused on a short segment (202 bp) of the D-loop, it is unlikely to fully reflect the range of genetic variability of this Critically Endangered species.
Methods
Sampling collection
We studied Nomascus hainanus at the Bawangling National Nature Reserve (19 N 02′~19 N 08′, 109 E 02′~109 E 13′), Hainan, China. Hainan gibbons vocalize almost every morning, and we monitored these vocalizations to identify group location (this was done for groups A, B, C, and D; we did not monitor group E). The total population size of the four monitored groups was 25 individuals.
Hainan gibbons are strictly arboreal and often travel in the uppermost regions of the tree canopy. Therefore, collecting noninvasive fresh fecal samples from all group members requires months of intense field observations. From March through August 2018, we spent five months following gibbon groups A, B, and C in order to obtain fresh fecal samples. Gibbon feces noticeably change color approximately two hours after defecation. We used this color change to only collect recently voided fecal samples (samples voided in the previous two hours). To avoid resampling the same individual, each fecal sample was scored for freshness (color), size, and shape. In those instances in which two or more fecal samples were located within a radius of 1.5 m, only one sample was collected. We used high temperature sterilized tweezers and petri dishes to collect the fecal samples. The samples were stored in liquid nitrogen, and then packed in dry ice and transported to the laboratory for cryogenic storage.
DNA extraction and detection
The average weight of a Hainan gibbon fecal clump is approximately 1000 mg. After carefully examining the fecal clump and judging it to be fresh, we extracted a 100–150mg sample from its interior or center. We extracted DNA from this 100–150mg sample using a QIAamp Fast DNA Stool Mini Kit following the manufacturer’s instructions. We avoided cross-contamination by using an ultra-clean workbench for extracting fecal genomic DNA. The extracted total DNA was subjected to 0.8% agarose gel electrophoresis, and contamination was monitored by including a negative extraction control (mock extraction submitted to PCR) per extraction. GreenView nucleic acid dye staining, and the estimated concentration and purity (260/280, 260/230 value) were recorded using a UV transilluminator. At the same time, we used a Qubit3.0 fluorescence quantifier to determine the concentration of DNA. In order to detect whether the extracted DNA concentration met the standard (≥50 ng/uL), we combined agarose gel electrophoresis, nucleic acid dye staining, and included a fluorescence quantifier to make to increase the reliability of the results.
Identification of polymorphic markers
Testing Potential Markers via amplification
We tested seven hypervariable segments of the Mitochondrial D-loop region primer pairs previously described as polymorphic (). Samples were amplified for each primer pair via PCR in a reaction volume of ∼10 uL containing 1 uL template DNA (≥50 ng/uL), 0.5 uL (10 pmol/uL) primer, 1 uL bovine serum albumin (New England BioLabs), 2 ul ddH2O, and 5 uL PCR Mix. The thermal profile for PCRs consisted of the following: denaturation and enzyme activation at 94 °C (3 min), 35 cycles of denaturation at 94 °C (30 s), annealing at 46–55 °C (30 s), extension at 72 °C (60 s) and final extension at 72 °C (10 min). PCR products were separated on 3% agarose gels by electrophoresis to visually assess the amplification efficiency, and set a negative PCR control in order to ensure amplifications were executed for Mitochondrial D-loop region of Hainan gibbon.
Table 1. Mitochondrial D-Loop region primer information.
Determination and proofreading of the target sequences
One pair of primers with the highest amplification rate and the longest fragment was selected from the initial 7 primer pairs and used for final target sequencing. The PCR products were sent for bidirectional sequencing to Kinco Biotech. Sequencing was performed on the ABI3730XL sequencer using the BigDye® Terminator v3.1 Cycle Sequencing Kit. The sequencing results were aligned in the GenBank sequence database using Blast software, in order to confirm that the amplification primer matched the target sequence. We used Clustal X (1.83) (Jeanmougin et al. Citation1998) for sequences alignment.
Data analysis
We evaluated the genetic diversity of the Hainan gibbon population by calculating Haplotype diversity (h) and Nucleotide diversity (π) using DnaSP 5.10 (Rozas and Rozas Citation1999). We assessed base composition and site variation using MEGA X (Kumar et al. Citation2001).
Results
DNA sample and identification of polymorphic markers
A total of 36 samples of Hainan gibbon feces were collected, six from Group A, 11 from Group B, and 19 samples from Group C (Supplementary Table S1). Excluding repeated samples (individual recognition results based on microsatellite markers, Guo et al. Citation2020) and unsuccessfully extracted DNA (<50 ng/uL), the 12 DNA samples used for PCR amplification included three individuals from Group A, four individuals from Group B, and five individuals from Group C.
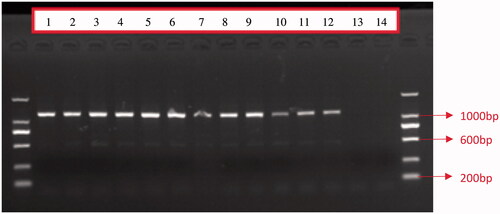
According to the success rate of amplification and the completeness of sequence information in the D-loop region and the sequencing results, primers L16007 and H00651 were selected. The PCR products were subjected to 1.5% agarose gel electrophoresis and a clear band (about1005 bp) was obtained ().
Haplotype distribution
All 12 sequences defined two distinct haplotypes. Individuals A01, A02, B06, C10, C06, and C19 shared a single haplotype, and individuals B01, C07, B07, B02, C08, and A04 shared a second haplotype. In the 12 samples, five polymorphic sites were detected. Nucleotide variation included conversions, transversions, insertions and deletions. Nucleotide polymorphic sites were primarily G to A conversions. The average content of T, C, A, and G bases in all sequences was 29.0%, 27.2%, 31.9% and 11.9%, respectively ().
Genetic diversity
The haplotype diversity (h) was 0. 545 and the nucleotide diversity (π) was 0. 000271.
Discussion
In the face of climate change, habitat degradation, forest fragmentation, and the conversion of natural landscapes for purposes of industrial agriculture to feed a growing human population, many animal species have experienced marked population decline (Estrada et al. Citation2020). A recent report of the United Nations Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services estimated that one million animal and plant species are threatened with extinction (IPBES Citation2019), and this includes some 65% of the over 500 species of nonhuman primates (Estrada et al. Citation2017; IUCN Citation2020). In order to better understand the population demography and population genetics of the Critically Endangered Hainan gibbon, our research team first conducted a long-term field investigation monitoring their behavior, ecology, group size, and group composition. In the present study we collected fresh fecal samples of 60% of the members of Group A (n = 3), 57% of the members of Group B (n = 4), and 50% of the membership of Group C (n = 5). The proportion of individuals sampled in our study is larger than in previous studies(Li et al. Citation2010; Bryant et al. Citation2016). In addition, we amplified the 1005 bp sequence of the mtDNA D-loop region, which is considerably longer than the 202 bp sequence analyzed in the only other study of mtDNA in this species (Li et al. Citation2010). Using a longer sequence is expected to improve the reliability of the Hainan gibbon genetic information.
The molecular markers identified in the mtDNA D-loop region of the last remaining population of Hainan gibbons suggest that the two haplotypes detected represent two ancestral female lineages. In response to rapid population decline and exceptionally small population size, the genetic diversity of this ape population remains extremely low. (Gibbs Citation2001; Püttker et al., Citation2008; Koskimäki et al. Citation2014).
This study represents the most comprehensive investigation of the genetic status of this rarest ape population. Our results indicate extremely low levels of polymorphism compared with available data on other wild primate populations, it also is among the lowest genetic variability reported for any highly isolated animal population (Newman et al. Citation2004; Hayaishi and Kawamoto Citation2006; Li et al. Citation2007; Liu et al. Citation2007; Li et al. Citation2010; Zhu et al. Citation2016). The low genetic diversity of the remaining Hainan gibbon population is consistent with their severe population decline (99.4%), that occurred over a 20–30 year period (Zhou Citation2008), which was the result of extreme deforestation and forest fragmentation that decreased their remaining area of suitable habitat from 27,784 km2 (Zhou et al. Citation2005) to approximately 16 km2 (Zhou Citation2008). The Hainan gibbon remains at extreme risk of extinction. Our findings reinforce the imperative to expand conservation efforts to protect the world’s last remaining Hainan gibbon population. We recommend a targeted conservation program of continuous population monitoring, regenerating native forests, strict enforcement against hunting, and obtaining genetic information on all remaining wild individuals. In the absence of an aggressive and comprehensive conservation management program, this rarest of ape species may not survive to the end of this century.
Author contributions
Zhou, J. designed the study; Guo Y.Q. wrote the manuscript and analyzed datas; Hai L completed experiments and analyzed datas; Paul A. Garber wrote the manuscript; Liu T and Li G conducted field investigations.
Supplemental Material
Download MS Word (14.1 KB)Acknowledgments
The authors thank Chrissie, Sara, Jenni, and Dax for their continued support and insights into the importance of kinship in understanding primate evolution.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in NCBI at https://www.ncbi.nlm.nih.gov/, reference number [MW052603, MW052604, MW052605, MW052606, MW052607, MW05260, MW052609, MW052610, MW052611, MW052612, MW052613, MW052615].
Additional information
Funding
References
- Andayani N, Morales JC, Michael RJ, et al. 2001. Genetic variability in mtDNA of the Silvery Gibbon: implications for the conservation of a critically endangered species [J]. Conservation Biology. 15(3): 770–775.
- Bryant JV, Gottelli D, Zeng X, Hong X, Chan BPL, Fellowes JR, Zhang Y, Luo J, Durrant C, Geissmann T, et al. 2016. Assessing current genetic status of the Hainan gibbon using historical and demographic baselines: implications for conservation management of species of extreme rarity. Mol Ecol. 25:3540–3556.
- Chan YC, Roos C, Inoue-Murayama M, Inoue E, Shih CC, Vigilant L. 2007. A comparative analysis of Y chromosome and mtDNA phylogenies of the Hylobates gibbons. BMC Evol Biol. 12:150, 23–27. doi:https://doi.org/10.1186/1471-2148-12-150.
- Cummins J. 2001. Mitochondrial DNA and the Y chromosome: parallels and paradoxes. Reprod Fertil Dev. 13(7–8):533–42. doi:https://doi.org/10.1071/rd01064.
- Deng HQ, Zhang MX, Zhou J. 2015. Recovery of the critically endangered Hainan gibbon Nomascus hainanus. Fauna & Flora International. 51:161–165.
- Estrada A, Garber PA, Chaudhary A. 2019. Expanding global commodities trade and consumption place the world’s primates at risk of extinction. PeerJ. 7:e7068.
- Estrada A, Garber PA, Chaudhary A. 2020. Current and future trends in socio-economic, demographic and governance factors affecting global primate conservation. PeerJ. 8:e9816.
- Estrada A, Garber PA, Mittermeier RA, Wich S, Gouveia S, Dobrovolski R, Nekaris KAI, Nijman V, Rylands AB, Maisels F, et al. 2018. Primates in peril: the significance of Brazil, Madagascar, Indonesia and the Democratic Republic of the Congo for global primate conservation. PeerJ. 6:e4869.
- Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, Di Fiore A, Nekaris KAI, Nijman V, Heymann EW, Lambert JE, et al. 2017. Impending extinction crisis of the world’s primates: why primates matter. Sci Adv. 3:e1600946.
- Gibbs JP. 2001. Demography versus habitat fragmentation as determinants of genetic variation in wild populations. Biol. Conserv. 100:15–20.
- Goossens B, Chikhi L, Ancrenaz M, Lackman-Ancrenaz I, Andau P, Bruford MW. 2006. Genetic signature of anthropogenic population collapse in orangutans. PLoS Biol. 4:e25–291.
- Guo Y, Chang J, Han L, Liu T, Li G, Garber PA, Xiao N, Zhou J. 2020. The genetic status of the critically endangered Hainan Gibbon (Nomascus hainanus): a species moving toward extinction. Front Genet. 11:608633.
- Guschanski K, Olivieri G, Funk SM, Radespiel U. 2007. MtDNA reveals strong genetic differentiation among geographically isolated populations of the golden brown mouse lemur. Microcebus ravelobensis. Conserv Genet. 8:809–821.
- Hayaishi S, Kawamoto Y. 2006. Low genetic diversity and biased distribution of mitochondrial DNA haplotypes in the Japanese macaque (Macaca fuscata yakui) on Yakushima Island. Primates. 47:158–164.
- IPBES. 2019. Intergovernmental science-policy platform on biodiversity and ecosystem services. https://www.ipbes.net/news/ipbes-global-assessment-summary-policymakers-pdf
- IUCN. 2020. IUCN Red List 2020. https://www.iucnredlist.org/search/stats?query=PRIMATES&searchType=species
- Jacob CD, Aralisa SG, Jurgi CA, Liliana CO, Ernesto RL, Leslie AK. 2014. Limited genetic diversity in the critically endangered Mexican howler monkey (Alouatta palliata mexicana) in the Selva Zoque. Mexico. Primates. 55:155–160.
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. 1998. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 23:403–405.
- Kocher TD, Kocher WK, Thomas AM, et al. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA. 1989. Vol. 86, pp. 6196–6200.
- Koskimäki J, Huitu O, Kotiaho JS, Lampila S, Mäkelä A, Sulkava R, Mönkkönen M. 2014. Are habitat loss, predation risk and climate related to the drastic decline in a Siberian flying squirrel population? A 15-year study. Popul Ecol. 56:341–348.
- Kuebler JD, Chen MH, Alexander ME, Rhodes J. 2016. Exercise Performance in Patients with D-Loop Transposition of the Great Arteries After Arterial Switch Operation: Long-Term Outcomes and Longitudinal Assessment. Pediatr Cardiol. 37(2), 283–289. doi:https://doi.org/10.1007/s00246-015-1275-5.
- Kumar S, Tamura K, Jakobsen IB, Nei M. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 17:1244–1245.
- Li M, Liu ZJ, Gou JX, Ren BP, Pan RL, Su YJ, Funk SM, Wei FW. 2007. Phylogeography and population structure of the golden monkeys (Rhinopithecus roxellana): inferred from mitochondrial DNA sequences. Am J Primatol. 69:1195–1209.
- Li G, Wei FW, Zhou J. 2010. Sequence analysis of mitochondrial D-loop region of Hainan gibbon and population rejuvenation. Biodiversity. 18:523–527.
- Liu ZJ, Ren BP, Wei FW, Long YC, Hao YL, Li M. 2007. Phylogeography and population structure of the Yunnan snub-nosed monkey (Rhinopithecus bieti) inferred from mitochondrial control region DNA sequence analysis. Mol Ecol. 16:3334–3349.
- Liu ZH, Yu SM, Yuan XC. 1984. The resource status of Hainan gibbon. Wildlife. 1–4.
- Newman TK, Jolly CJ, Rogers J. 2004. Mitochondrial phylogeny and systematics of baboons (Papio). Am J Phys Anthropol. 124:17–27.
- Püttker T, Meyer-Lucht Y, Sommer S. 2008. Fragmentation effects on population density of three rodent species in secondary Atlantic Rainforest, Brazil. Stud. Neotrop. Fauna Environ. 43:11–18.
- Rozas J, Rozas R. 1999. DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics. 15:174–175.
- Su B, Monda K, Wang W. 1995. Molecular phylogeny of Chinese concolor gibbons (subgenus Nomascus) using noninvasive DNA genotyping. In: Xia W, Zhang , editors. Primate research and conservation. Beijing: China Forestry Publishing House; p. 55–63.
- Thinh VN, Mootnick AR, Geissmann T, Li M, Ziegler T, Agil M, Moisson P, Nadler T, Walter L, Roos C. 2010a. Mitochondrial evidence for multiple radiations in the evolutionary history of small apes. BMC Evol Biol. 10:74
- Thinh VN, Rawson B, Hallam C, Kenyon M, Nadler T, Walter L, Roos C. 2010b. Phylogeny and distribution of crested gibbons (genus Nomascus) based on mitochondrial cytochrome b gene sequence data. Am J Primatol. 72:1047–1054.
- Thomas O. 1892. XXIII.–Note on the Gibbon of the Island of Hainan (Hylobates hainanus sp. n. ). J Nat Hist. 9:145–146.
- Whittaker DJ, Morales JC, Melnick DJ. 2007. Resolution of the Hylobates phylogeny: congruence of mitochondrial D-loop sequences with molecular, behavioral, and morphological data sets. Mol Phylogenet Evol. Nov;45(2):620–8. doi:https://doi.org/10.1016/j.ympev.2007.08.009.
- Zhang Y. 1995. A molecular phylogeny of gibbons based on DNA sequences. In: Xia W, Zhang Y, editors. Primate research and conservation. Beijing: China Forestry Publishing House; p. 50–54.
- Zhou J. 2008. The ecological and behavioral characteristics of Hainan black-crowned gibbon. Changchun: Northeast Normal University.
- Zhou J, Wei F, Li M, Zhang J, Wang D, Pan R. 2005. Hainan black-crested gibbon is headed for extinction. Int J Primatol. 26:453–465.
- Zhu WL, Cai JH, Chen JL. 2016. Polymorphism analysis of the cytochrome b gene and D-loop sequence of Japanese monkeys. J Biol. 33:20–23.