Abstract
The mitogenome of Ripeacma umbellata Wang, 2009 was reported in this study. It was 15,486 bps long and strongly AT biased, consisting of 13 protein-coding genes (PCGs), 22 transfer RNAs (tRNAs), 2 ribosomal RNAs (rRNAs), and 1 non-coding control region (351 bp). Most PCGs used the conventional ATN start codon, except for cox1 and cox2. Four genes used single T residue as stop codon rather than the routinely used TAA or TAG. All tRNAs, except for TrnS1, could fold into the cloverleaf secondary structure. Bayesian inference phylogenetic tree built on 13 PCGs from R. umbellata and another 21 species in Gelechioidea demonstrated that genus Ripeacma was a member in Autostichidae, which was consistent with the latest phylogenetic study.
The small moth Ripeacma umbellata Wang, Citation2009 belongs to the genus Ripeacma Moriuti, Saito & Lewvanich (Autostichidae, Lepidoptera), which currently contains 34 species in Palearctic and Oriental regions (Li and Wang Citation2017; Kim and Lee Citation2017). The systematic position of Ripeacma within the superfamily Gelechioidea had long been a problem to all the involved researchers (e.g. Lvovsky Citation2005, 2015; Heikkilä et al. Citation2014; Kim and Lee Citation2017), until a recent molecular study seemed to have given it a solid answer, that Ripeacma in nature belonged to the family Autostichidae (Wang and Li Citation2020). In attempt to test this scientific assertion, we herein sequenced for the first time the mitogenome of a Ripeacma member, R. umbellata and used it as the representative to recover the phylogenetic relationship of this genus in Gelechioidea. The moths were collected from Maolan Natural Reserve (25°17′10″N, 108°42′42″E), Guizhou, China in 2020, using light trap. The specimens were then stored in absolute alcohol under −20 °C in the Morphological Laboratory of Guizhou University of Traditional Chinese Medicine, Guiyang, China (Aihui Yin, [email protected]) under the voucher number GZUTCM:M24–27.
The next-generation sequencing (NGS) was performed using Illumina HiSeq2500 platform in Sangon Biotech (Shanghai) Co., Ltd., China. The genome assembly and gap-filling were carried out with SPAdes version 3.14.1 (https://github.com/ablab/spades) (Bankevich et al. Citation2012), ARC version 1.1.3 (https://github.com/ibest/ARC) (Hunter et al. Citation2015), BWA version 0.7.17 (https://sourceforge.net/projects/bio-bwa/) (Li Citation2013), and samtools version 0.1.19 (https://github.com/samtools/samtools) (Li et al. Citation2009). Sequence polish was aided with Pilon version 1.23 (https://github.com/broadinstitute/pilon) (Walker et al. Citation2014). MITOS WebServer (http://mitos2.bioinf.uni-leipzig.de/index.py) was utilized for annotation.
The 15,486 bp long circular mitogenome of R. umbellata (GenBank: MW366997) was constructed. It was strongly AT biased (AT 79.9%, CG 20.1%), and shared the typical set of genes (13 protein-coding genes [PCGs], 22 transfer RNAs [tRNAs], and 2 ribosomal RNAs [rRNAs]) with other metazoan animals (Wolstenholme Citation1992). Most PCGs of R. umbellata used typical start codon ATN at initiation, only cox1 and cox2 started with unorthodox codons CGA and TTG respectively. In terms of stop codon, most PCGs used the routine TAA or TAG, except for cox1, cox2, nad4, and nad5, which used a single T residue to stop transcription. All tRNAs could fold into the clover-leaf secondary structure, excluding TrnS1, due to its lack of the dihydrouracil arm. The special tRNA gene order TrnM-TrnI-TrnQ which was shared among mitogenomes of almost all Ditrysian moths was also observed in R. umbellata (Cao et al. Citation2012; Park et al. Citation2016). The putative A + T rich control region was 351 bps in length, and had very high AT ratio (93.2%).
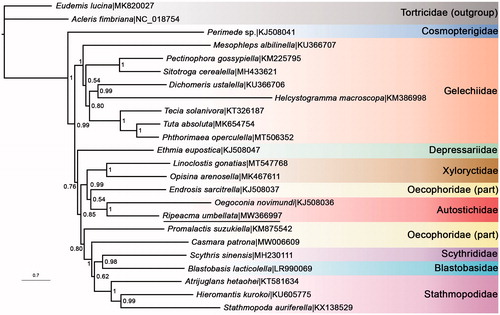
MrBayes version 3.2.7 (http://nbisweden.github.io/MrBayes/sourseforge) (Ronquist et al. Citation2012) was run for the data set built of 13 PCGs from R. umbellata plus another 21 species in Gelechioidea to generate a BI phylogenetic tree using ‘GTR + I + G’ substitution model (). The families Autostichidae, Gelechiidae, Stathmopodidae, and Xyloryctidae that had multiple representatives were successfully recovered as monophyletic. However, Oecophoridae appeared to be polyphyletic in this study. R. umbellata was indeed a member of the family Autostichidae as illustrated by Wang and Li (Citation2020).
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/nuccore/MW366997 under the accession no. MW366997.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19:455–477.
- Cao YQ, Ma C, Chen JY, Yang DR. 2012. The complete mitochondrial genomes of two ghost moths, Thitarodes renzhiensis and Thitarodes yunnanensis: the ancestral gene arrangement in Lepidoptera. BMC Genomics. 13:276.
- Heikkilä M, Mutanen M, Kekkonen M, Kaila L. 2014. Morphology reinforces proposed molecular phylogenetic affinities: a revised classification for Gelechioidea (Lepidoptera). Cladistics. 30(6):563–589.
- Hunter SS, Lyon RT, Sarver BAJ, Hardwick K, Forney LJ, Settles ML. 2015. Assembly by reduced complexity (ARC): a hybrid approach for targeted assembly of homologous sequences. Biorxiv. 014662.
- Kim SR, Lee SH. 2017. First review of subfamily Periacminae (Lepidoptera: Xyloryctidae s.l.) from Korea: newly recorded genus including two new descriptions. J Asia-Pac Entomol. 20(2):387–394.
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv. 13033997.
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics. 25:2078–2079.
- Li SR, Wang SX. 2017. Description of six new species of the genus Ripeacma (Lepidoptera: Oecophoridae) from China, with a checklist of the world species. Zootaxa. 4268(2):270–284.
- Lvovsky AL. 2005. Periacmini, a new tribe of the microlepidopteran subfamily Amphisbatinae (Lepidoptera, Amphisbatidae). Entomol Rev. 85(1):91–92.
- Lvovsky AL. 2015. Composition of the subfamily Periacminae (Lepidoptera, Lypusidae) with descriptions of new and little known species of the genus Meleonoma Meyrick, 1914 from South, East, and South-East Asia. Entmol Rev. 95(6):766–778.
- Park JS, Kim MJ, Jeong SY, Kim SS, Kim I. 2016. Complete mitochondrial genomes of two gelechioids, Mesophleps albilinella and Dichomeris ustalella (Lepidoptera: Gelechiidae), with a description of gene rearrangement in Lepidoptera. Curr Genet. 62:809–826.
- Ronquist F, Teslenko M, Mark PV, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542.
- Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, et al. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 9(11):e112963.
- Wang QY, Li HH. 2020. Phylogeny of the superfamily Gelechioidea (Lepidoptera: Obtectomera), with an exploratory application on geometric morphometrics. Zool Scr. 49(3):307–328.
- Wolstenholme DR. 1992. Animal mitochondrial DNA: structure and evolution. Int Rev Cytol. 141(6):173–216.
- Yuan GX, Wang SX. 2009. A new species of Ripeacma from Hong Kong (Lepidoptera: Oecophoridae). Entomol News. 120(4):449–452.