Abstract
Chimonobambusa hejiangensis is of the unique edible bamboo specie of high quality in China. We studied the complete chloroplast(cp) genome of C. purpurea in this study. The cp genome of C. hejiangensis (GenBank accession: MW186792) was 138,911 bp in length, including a large single-copy (LSC) region of 82,498 bp, a small single-copy (SSC) region of 12,743 bp and a pair of inverted repeated (IR) regions of 21,835 bp. The genome contained 133 genes, including 86 protein-coding genes, 39 tRNA genes, and 8 rRNA genes. Based on 39 cp genomes, we used the phylogenetic analysis to build phylogenetic tree, indicating that C. hejiangensis is closely related to C. tumidissinoda. Also, the phylogenetic relationship of lineages might be (Hsuehochloa + (((Shibataea clade + Arundinaria clade) + Indocalamus wilsonii) + ((Bergbambos + Indocalamus) + (((African alpine bamboos + Gaoligongshania) + (Chimonocalamus + Kuruna))+(Thamnocalamus + Phyllostachys clade))))). It could be devoted to phylogenetic analysis of Arundinarieae.
Chimonobambusa hejiangensis C. D. Chu & C. S. Chao is one of the unique edible bamboo species of high quality in China, which is only natural distributed in Sichuan and Guizhou Provinces (Li and Stapleton Citation2006; Ma et al. Citation2016; Wang et al. Citation2019). With aerial roots, high ornamental value, crispy and delicious bamboo shoots and high nutritional value, C. hejiangensis is deeply loved by consumers all over China and Southeast Asia, and its economic benefits are very significant (Wang et al. Citation2016). In this study, we report and characterize the chloroplast genome of C. hejiangensis. We reconstruct a phylogenetic tree to reveal the relationship and provide useful information for further study of C. hejiangensis.
The materials of C. hejiangensis were collected from Chengdu City, Sichuan Province, China (N30°40′49″, E103°43′53″) on 10 June 2020. The voucher specimens were deposited at the Herbarium of Chengdu Academy of Agricultural and Forestry Sciences (http://www.cdnky.com) under the voucher number LYK20200610-05. The chloroplast DNA was extracted from fresh leaves of an individual of C. hejiangensis. Total DNA was used to generate libraries with an average insert size of 400 bp, sequenced using the Illumina HiSeq platform, generating 150 bp paired-end reads. Ten million high-quality reads were mapped to the published C. tumidissinoda chloroplast genomes as references using MUMmer v3.1 (Kurtz et al. Citation2004). We used A5-miseq v20150522 (Coil et al. Citation2015) and SPAdes v3.9.0 (Bankevich et al. Citation2012) to assemble these reads into complete chloroplast genomes. The assembled chloroplast genome sequence was annotated using the online Geseq web server (https://chlorobox.mpimp-golm.mpg.de/geseq.html).
The complete plastid genome sequence of C. hejiangensis (GenBank accession: MW186792) was 138,911 bp in length, including a large single-copy (LSC) region of 82,498 bp, a small single-copy (SSC) region of 12,743 bp and a pair of inverted repeated (IR) regions of 21,835 bp. The complete chloroplast genome contained 133 genes, including 86 protein-coding genes, 39 tRNA genes and 8 rRNA genes. The complete genome GC content was 38.92%, and the corresponding values of the LSC, SSC and IR were 37.01%, 33.20% and 44.21%, respectively.
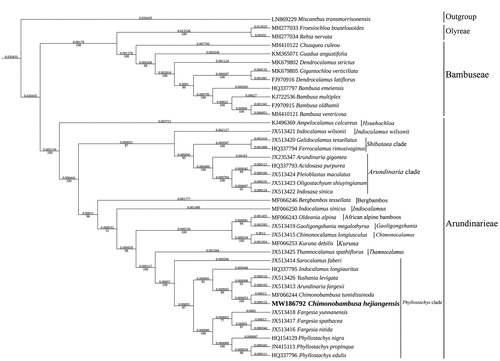
The maximum-likelihood phylogenetic tree was constructed based on 39 complete chloroplast genomes of Bambusoideae species, and Miscanthus transmorrisonensis (Panicoideae) as outgroup. All of them were downloaded from NCBI GenBank. The sequences were aligned using MAFFT v7.037 (Katoh and Standley Citation2013), and the phylogenetic tree constructed using IQ-TREE Multicore version 1.6.12 (Gao et al. Citation2018). Based on the phylogenetic tree, we can see that C. hejiangensis has a close relationship with C. tumidissinoda. Also, the results supported that Gelidocalamus tessellatus belongs to Shibataea clade and the phylogenetic relationship of lineages might be (Hsuehochloa + (((Shibataea clade + Arundinaria clade) +Indocalamus wilsonii) + ((Bergbambos + Indocalamus) + (((African alpine bamboos+Gaoligongshania)+(Chimonocalamus + Kuruna))+(Thamnocalamus+Phyllostachys clade))))) (Ma et al. Citation2014, 2017; Zhang et al. Citation2020). It could be devoted to the phylogenetic analysis of Arundinarieae ().
Figure 1. Maximum-likelihood phylogenetic analysis of 39 species of Bambusoideae and Miscanthus transmorrisonensis (Panicoideae) as outgroup based on plastid genome sequences by IQ-TREE multicore version 1.6.12 under GTR + R6 model for 5000 ultrafast bootstraps. Branch lengths (above) and bootstrap values (below) were indicated around nodes. GenBank accession numbers of each species were listed in the phylogenetic tree.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession No. MW186792. The associated “BioProject”, “SRA”, and “BioSample” numbers are PRJNA684970, SRR13258744, and SAMN17073575, respectively.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477.
- Coil D, Jospin G, Darling AE. 2015. A5-MiSeq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics. 31(4):587–589.
- Gao FL, Shen JG, Liao FR, Cai W, Lin SP, Yang HK, Chen SL. 2018. The first complete genome sequence of narcissus latent virus from Narcissus. Arch Virol. 163(5):1383–1386.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5(2):R12.
- Li DZ, Stapleton C. 2006. Flora of China 22. Beijing, Science Press. p. 152–161.
- Ma PF, Vorontsova MS, Nanjarisoa OP, Razanatsoa J, Guo ZH, Haevermans T, Li DZ. 2017. Negative correlation between rates of molecular evolution and flowering cycles in temperate woody bamboos revealed by plastid phylogenomics. BMC Plant Biol. 17(1):260.
- Ma PF, Zhang YX, Zeng CX, Guo ZH, Li DZ. 2014. Chloroplast phylogenomic analyses resolve deep-level relationships of an intractable bamboo tribe Arundinarieae (Poaceae). Syst Bidl. 63(6):933–950.
- Ma S, Sun QL, Gou GQ. 2016. Study on the growth of Chimonobambusa hejiangensis (Poales: Poaceae). J MT Agric Biol. 35(1):71–73.
- Wang GJ, Chen H, Ma GL, Li CX, Wang XJ, Yang DS, Wang R. 2016. Research on the law and influence factors of shoot degeneration for Chimonobambusa hejiangensis. World Bamb Ratt. 14(1):1–9.
- Wang XJ, Wang GJ, Ma GL, Chen H. 2019. Prediction for potential distribution area of Chimonobambusa hejiangensis in China based on GIS and MaxEnt Model. World Bamb Ratt. 17(5):9–15. 26.
- Zhang YX, Guo C, Li DZ. 2020. A new subtribal classification of Arundinarieae (Poaceae, Bambusoideae) with the description of a new genus. Plant Div. 42(3):127–134.
