Abstract
The first complete plastid genome (plastome) of a small genus, Tibetia (Ali) H. P. Tsui, was sequenced from T. liangshanensis P. C. Li. The total genome size of T. liangshanensis was 123,372 bp in length, containing 76 protein-coding genes, 30 tRNAs genes, and four rRNAs. The genome lacked an inverted repeat (IR) region. Its rpl22 and rps16 genes were absent and clpP gene lost two introns. The overall GC content was 34.68%. Phylogenetic analysis of the plastome of T. liangshanensis with other legumes confirmed its phylogenetic position.
Tibetia (Ali) H.P. Tsui is an endemic genus of about 5 species distributed in the Himalayan region and the Qinghai-Tibetan Plateau (QTP) of southwestern China. Tibetia liangshanenis P.C. Li is an endemic species in the Hengduan Mountains of SW China and previous morphological (Xie et al. Citation2012) and phylogenetic analysis (Xie et al. Citation2016) suggest its relationship with several Tibetia species and genera in the inverted repeat lacking clade (IRLC) clade in Papilionoideae. In this study, we report the complete chloroplast genome (plastome) of this species, which will provide valuable resources for further studies on genetic diversity, species delimitation within Tibetia, and structural plastome and phylogenetic studies in Leguminosae.
Fresh leaves of T. liangshanensis were collected from Liangshan County, Sichuan province of China (27°50′3.50′′N and 102°33′9.10′′E). The voucher specimens were deposited in the herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (http://www.kun.ac.cn/, Tao Deng, [email protected]) under the voucher number Liuj153027. Total genomic DNA (gDNA) was isolated with a modified CTAB protocol (Doyle JJ and Doyle JL Citation1987) and send to Beijing Genomics Institute (BGI), Shenzhen, China for library construction and sequencing. Illumina HiSeq2000 platform was used for paired-end (PE) reads generation preparation with 2 × 150 bp PE reads. Libraries were size selected for 350 bp inserts. The plastome sequence of the peanut (Arachis hypogaea L.) was downloaded from GenBank (NC_026676) and used as the reference plastome. The raw data were filtered and the plastome was assembled following by Zhang et al. (Citation2020). Contigs were then connected into plastome using Bandage Ubuntu dynamic version 8.0 (Wick et al. Citation2015). Finally, PE reads were mapped to the plastome using Bowtie2 (Langmead and Salzberg Citation2012) implemented in Geneious version 9.1.4 (Kearse et al. Citation2012) to determine if there were any differences. Annotation was performed using GeSeq (https://chlorobox.mpimp-golm.mpg.de/cite-geseq.html) (Tillich et al. Citation2017), coupled with manual adjustment in Geneious. The online tRNAscan-SE Search Service (Schattner et al. Citation2005) was used to determine tRNAs. The final complete plastomes were deposited into GenBank with accession number MF193597 for T. liangshanensis.
The complete plastome of T. liangshanensis was 123,372 bp in length and showed inverted repeat (IR) lacking plastome structures as other IRLC species (Tillich et al. Citation2017). It comprised of 110 unique genes including 76 protein-coding genes, 30 tRNAs, and four rRNAs. The overall GC content was 34.68%. Similar to other IRLC plastomes. T. liangshanensis lost rpl22 and rps16 gene. In addition, its clpP gene lost two introns. Protein coding regions (PCRs) were 66,108 bp long, representing 53.6% of the complete chloroplast genome. Contrary to non-IRLC Ammopiptanthus mongolicus (78,945 bp/153,935 bp = 51.0%), the percentage of T. liangshanensis’s PCR was higher.
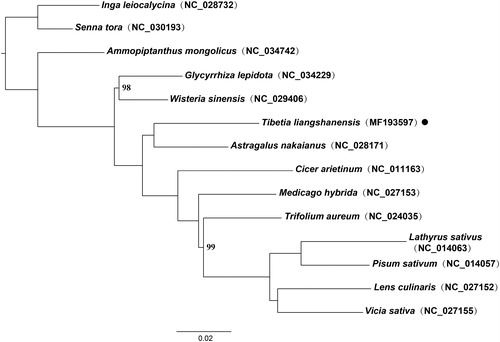
A phylogenetic tree was constructed using RAxML version 8.2.12 (Stamatakis Citation2014) based on 14 plastomes of Fabaceae. Senna tora (L.) Roxb. and Inga leiocalycina Benth. were used as outgroups. The phylogenetic results showed that Tibetia liangshanensis nested within the IRLC and formed a clade with Astragalus nakaianus with 100% bootstrap values (). The complete plastome of T. liangshanensis will provide a valuable basis for further study on genetic diversification, evolution, and plastome pattern of legumes.
Acknowledgments
We thank Doc. Jie Liu from Kunming Institute of Botany, Chinese Academy of Sciences for the collection of materials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data of T. liangshanensis are openly available in GenBank of NCBI at [https://www.ncbi.nlm.nih.gov] (https://www.ncbi.nlm.nih.gov/) under the accession no. MF193597, the associated BioProject, SRA, and Bio-Sample numbers are PRJNA725310, SRR14338556, and SAMN18875862, respectively.
Additional information
Funding
References
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin. 19:11–15.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649.
- Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods. 9(4):357–359.
- Schattner P, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33:W686–W689.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq – versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–W11.
- Wick RR, Schultz MB, Zobel J, Holt KE. 2015. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics. 31(20):3350–3352.
- Xie YP, Meng Y, Sun H, Nie Z-L. 2012. Pollen morphology of Tibetia (Fabaceae) from the Hengduan mountains, with emphasis on the taxonomical status of Tibetia liangshanensis. Plant Diversity Resour. 34(4):326–332.
- Xie Y-P, Meng Y, Sun H, Nie Z-L. 2016. Molecular phylogeny of Gueldenstaedtia and Tibetia (Fabaceae) and their biogeographic differentiation within Eastern Asia. PLOS One. 11(9):e0162982.
- Zhang R, Wang Y-H, Jin J-J, Stull GW, Bruneau A, Cardoso D, De Queiroz LP, Moore MJ, Zhang S-D, Chen S-Y, et al. 2020. Exploration of plastid phylogenomic conflict yields new insights into the deep relationships of Leguminosae. Syst Biol. 69(4):613–622.