Abstract
Pleione maculata is an epiphytic orchid with significant ornamental value and medicinal value. Here, we report the first complete chloroplast genome of P. maculata. The circular genome was 158,394 bp in length and consisted of a pair of inverted repeats (IR 26,646 bp), which were separated by a large single copy region (LSC 86,603 bp) and a small single copy region (SSC 18,499 bp). It contained 135 genes, including 89 protein-coding genes, 38 tRNAs and 8 rRNAs. Phylogenetic analysis of cp genomes from 41 species of Orchidaceae revealed that all species of Pleione formed one monophyletic clade and P. maculata was located at the base of the genus with high bootstrap values (≥99.1%).
The genus Pleione D. Don (Orchidaceae), comprising about 33 speices (7 natural hybrid species) of terrestrial, lithophytic or epiphytic orchids, is widely distributed in China, Vietnam, India, Bhutan, Nepal, Thailand, and Myanmar (Chen et al. Citation2009; Govaerts et al. Citation2016). Pleione are fairly popular ornamental plants in Europe and the USA and widely used as traditional medicine in Asian countries (Teoh Citation2016). Pleione bulbocodioides (Franchet) Rolfe and P. yunnanensis (Rolfe) Rolfe are also the original plant of traditional Chinese medicine Shān Cí Gū. The pseudobulb was called commonly ‘ice ball’, which has the functions of clearing heat, detoxifying, and resolving phlegm (You et al. Citation2011). Pleione is also a world-famous ornamental, with showy, color-rich and leafless flowers in flowering period (Zhang et al. Citation2020).
Pleione maculata (Lindley) Lindley & Paxton, distributed in Bhutan, India, Myanmar, Nepal, Thailand and China between 600 and 1600 meters above sea level, is an epiphytic herb growing on tree trunks and mossy rocks in broad-leaved forests (Chen et al. Citation2009). Pseudobulbs of P. maculata, has commonly been applied in the northeast India for the treatment of cuts, wound, or liver complaints (Simpli et al. Citation2018). In the wild, P. maculata is one of the most important parents for a succession of hybrids (Gravendeel et al. Citation2004; Chen et al. Citation2009). Although Pleione have high ornamental value and medicinal value, there are a few reports on the chloroplast genome. So far, all species with cp genomes reported belong to the Sect. Humiles, such as, P. bulbocodioides (Shi et al. Citation2018), P. chunii C. L. Tso (Wu et al. Citation2019), P. formosana Hayata (Jiang et al. Citation2019), P. forrestii Schltr. (Wu et al. Citation2019) and P. pleionoides (Kraenzl. ex Diels) Braem et H. Mohr (Chen et al. Citation2019). In this study, we assembled and characterized the complete chloroplast of P. maculata to provide a better understanding of the phylogeny and genetics of genus Pleione.
Fresh leaves of P. maculata were collected from the individual growing in the greenhouse of Yunnan Normal University, Kunming, China (24°52'1.41’N, 102°51'19.39’E), and voucher specimen (OP-001) deposited at Herbarium of Yunnan Normal University (YNUB). Total genomic DNA was extracted from fresh leaves using a modified CTAB method (Allen et al. Citation2006) and sequenced by the Illumina Hiseq 2000 sequencing platform (Illumina, CA, USA) at Novogene (Beijing, China). In total, 3.44 GB of raw data with 11,464,366 raw reads was obtained. Raw reads were filtered by NGS QC Toolkit (Patel et al. Citation2012). The plastome was de novo assembled using NOVOPlasty (Dierckxsens et al. Citation2017). After assembled, the genome was automatically annotated using DOGMA (Wyman et al. Citation2004), then adjusted with Geneious Prime 2020.0.3 (https://www.geneious.com) and submitted to GenBank with accession number MW699846.
The length of the genome sequence of P. maculata was 158,394 bp, and it was a typical quadripartite structure. It contained a large single copy (LSC) region (86,603 bp), a small single copy (SSC) region (18,499 bp), and two reverse sequence repeats (IR) regions (26,646 bp). The content of GC in whole chloroplast genome was 37.3%, including 35.2% in LSC region, 30.4% in SSC region and 43.2% in IR region. A total of 135 genes were contained, including 89 protein-coding genes, 38 tRNA genes and 8 rRNA genes. Among them atpF, ndhA, ndhB, rpl2, rpoC1, rps12, rps16, trnA-UGC, trnG-GCC, trnI-CAU, trnI-GAU, trnK-UUU, trnL-UAA, trnV-UAC contain a single intron, and two introns are contained in clpP and ycf3.
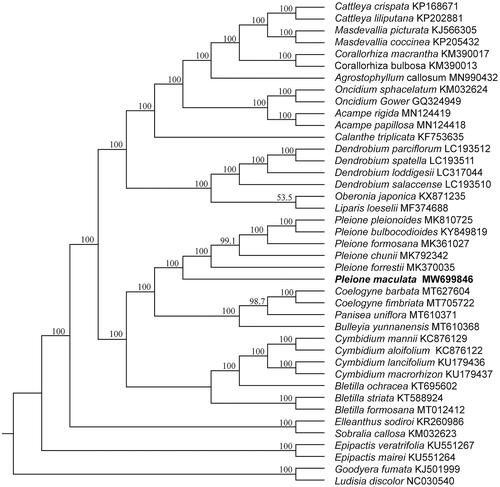
To clarify the phylogenetic position of P. maculata, 41 published chloroplast genomes from Orchidaceae were aligned by using MAFFT 7.308 (Katoh and Standley Citation2013) with Goodyera fumata (GenBank accession KJ501999) and Ludisia discolor (GenBank accession NC030540) as outgroups. Maximum-likelihood (ML) tree was constructed using RAxML 8.2.11 (Stamatakis Citation2014) with the GTR + G nucleotide substitution model. The branch supports were computed with 1000 bootstrap replicates. The phylogenetic tree showed that six species of Pleione formed one monophyletic clade with 100% bootstrap value () and P. maculata was located at the base of the genus with high bootstrap value (≥99.1%). The complete chloroplast genome of P. maculata will provide useful resource for identification, conservation, and utilization of this valuable species. Moreover, it will be helpful to better understand the phylogeny of genus Pleione and even the family Orchidaceae.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at [https://www.ncbi.nlm.nih.gov/] (https://www.ncbi.nlm.nih.gov/) under the accession number MW699846. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA726024, SRR14354833, and SAMN18918057, respectively.
Additional information
Funding
References
- Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF. 2006. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc. 1(5):2320–2325.
- Chen JL, Dai ZW, Dai XY, Lan SR, Wu SS. 2019. The complete chloroplast genome of Pleione pleionoides (Orchidaceae). Mitochondrial DNA Part B. 4(2):2167–2168.
- Chen XQ, Cribb PJ, Gale SW. 2009. Pleione D. Don. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China vol. 25. Beijing: Science Press; St. Louis, MO: Missouri Botanical Garden Press; p. 325–333.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18.
- Govaerts R, Campacci MA, Baptista DH. 2016. World checklist of Orchidaceae. Kew: The Board of Trustees of the Royal Botanic Gardens. http://apps.kew.org/wcsp.
- Gravendeel B, Eurlings MCM, van den Berg C, Cribb PJ. 2004. Phylogeny of Pleione (Orchidaceae) and parentage analysis of its wild hybrids based on plastid and nuclear ribosomal ITS sequences and morphological data. Syst Bot. 29(1):50–63.
- Jiang MT, Chao WC, Huang CL, Lan SR, Liu ZJ, Wu SS. 2019. The complete chloroplast genome of Pleione formosana (Orchidaceae). Mitochondrial DNA Part B. 4(1):1044–1046.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Patel RK, Jain M, Liu ZJ. 2012. NGS QC toolkit: a toolkit for quality control of next generation sequencing data. PLOS One. 7(2):e30619.
- Shi YC, Yang LF, Yang ZY, Ji YH. 2018. The complete chloroplast genome of Pleione bulbocodioides (Orchidaceae). Conserv Genet Resour. 10(1):21–25.
- Simpli HD, Choudury MG, Borah VV. 2018. A review of the unexplored medicinal orchids of the genus Pleione. MIOS. 19(8):3–12.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Teoh ES. 2016. Medicinal orchids of Asia. Cham (Switzerland). Springer International Publishing.
- Wu SS, Shen LM, Ling R, Dai ZW, Liu ZJ, Lan SR. 2019. Next-generation sequencing yields the complete chloroplast genome of Pleione chunii, a vulnerable orchid in China. Mitochondrial DNA Part B. 4(2):2576–2578.
- Wu SS, Wu XQ, Dong N, Ling R, Jiang H. 2019. Next-generation sequencing yields the complete chloroplast genome of Pleione forrestii (Orchidaceae). Mitochondrial DNA Part B. 4(2):2777–2778.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20(17):3252–3255.
- You C, Song XJ, Shi CJ. 2011. Effects of different culture media on seed germination of Pleione bulbocodioides (Franch.) Rolfe. Med Plant. 2(10):18–20.
- Zhang W, Wang JH, Fan ZX, Zhang SB. 2020. Research Progress on Cross Breeding of Pleione (Orchidaceae). Northern Horticulture. 44(14):136–144.