Abstract
Cardamine fallax (O. E. Schulz) Nakai. is a perennial plant distributed in Eastern Asia. However, no extensive genomic studies are available on C. fallax. In this paper, the authors describe the complete chloroplast (cp) genome of C. fallax and its phylogenetic analysis. The cp genome is 154,797 bp in length with 36.3% GC content and consists of a pair of inverted repeats (IRs) of 26,521 bp that separated a large single-copy (LSC) region of 83,817 bp and a small single-copy (SSC) region of 17,938 bp. It was found to contain 113 unique genes, of which 80 were protein-coding genes, 29 were transfer RNAs, and four were ribosomal RNAs. Also, six PCGs, eight tRNA and four rRNA genes were duplicated in the IR region and one gene as a pseudogene. Phylogenetic analysis showed that all Cardamine species are highly conserved, and C. fallax was associated with the sister clade C. amaraeformis and C. parviflora.
The genus Cardamine, one of the most extensive genera of the Brassicaceae family, includes more than 200 species dispersed worldwide (Al-Shehbaz Citation1988; Lihova and Marhold Citation2006). The species C. fallax (O. E. Schulz) Nakai. was considered as C. flexuosa subsp. fallax O. E. Schulz. Some researchers presently distinguish it as a distinct species (Kitagawa Citation1982; Kudoh et al. Citation1993) or as a subspecies (Kimata Citation1983) or variety (Cheo et al. Citation1987; Lee Citation1996) of C. flexuosa. Nevertheless, in the latest Flora of China (Zhou et al. Citation2001) and Flora of Japan (Al-Shehbaz et al. Citation2006), the C. fallax and C. flexuosa subsp. fallax has been included in the synonymy of C. parviflora L. However, the main difference between these two species is the shape of cauline leaves (Marhold et al. Citation2007). Several molecular phylogenetic analyses have been studied with both nrDNA internal transcribed spacer (ITS) and cpDNA sequences to understand the phylogenetic relationship of the species C. fallax (Marhold et al. Citation2007) that provided contradictory results. However, recent studies have shown that the plastid genome is highly conserved, which helps resolve taxonomic and phylogenetic implications (Lihova and Marhold Citation2006). Therefore, in the current study, we described the characteristics of the complete cp genome of C. fallax, and phylogenetic analyses might reveal the phylogenetic position of C. fallax in the Brassicaceae clade.
The young, fresh C. fallax (O. E. Schulz) Nakai. leaf materials were collected from mountain Cheongok, Bonghwa-gun, South Korea (geospatial coordinates: N37°4′9″, E128°57′47″) and the voucher specimen (YNUH21C063) was deposited in the Yeungnam University Plant Herbarium, Gyeongsan, South Korea (Prof. SeonJoo Park, [email protected]). Whole genomic DNA was obtained by using a modified cetyltrimethylammonium bromide method (Doyle Citation1990) and stored at the DNA Bank, Department of Life Sciences, Yeungnam University, Gyeongsan, South Korea (Prof. SeonJoo Park, [email protected]). Whole-genome sequencing was accomplished with a pair-end library (150 × 2), and an insert size of 350 base pairs (bp) using the Illumina HiSeq-2500 sequencing system at LabGenomics, Seongnam, South Korea. Read quality was assessed with FastQC v0.11.9 (Andrews Citation2010), and low-quality reads were trimmed with Trimmomatic 0.40 (Bolger et al. Citation2014). The following clean reads were filtered using the GetOrganelle v1.7.4.1 pipeline (https://github.com/Kinggerm/GetOrganelle) to obtain plastid-like reads and the filtered reads were assembled de novo method using SPAdes v3.15.2 (Prjibelski et al. Citation2020). Gene annotation was performed using Geneious Prime v2021.1.1. A circular cp genome map was generated with OrganellarGenome DRAW (OGDRAW) (Greiner et al. Citation2019). The complete cp genome sequence of C. fallax and its gene annotation was submitted to GenBank (MZ043778).
The C. fallax (O. E. Schulz) Nakai. cp genome size was 154,797 bp with a similar quadripartite structure, containing a large single-copy region (LSC, 83,817 bp) and a small single-copy region (SSC, 17,938 bp), divided by a pair of inverted repeats (IRs, 26,521 bp). This genome encoded a total of 113 unique genes, of which 18 were duplicated in the IR regions. Of the 113 genes, 80 were protein-coding genes (PCGs), 29 were tRNA genes, and four were rRNA genes. Of these, 14 genes contained one intron (eight protein-coding and six tRNA genes) and three encoded two introns (clpP, ycf3 and rps12). The rps12 gene was a trans-spliced gene with its 5′-end exon located in the LSC region and its intron 3′-end exon duplicated in IR regions. The total GC content of the cp genome was 36.3%.
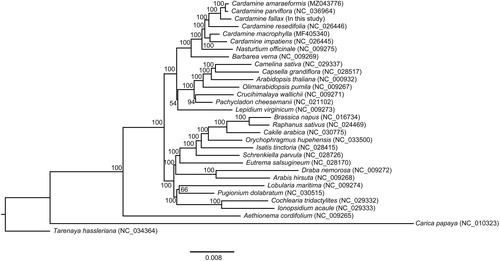
To study the phylogenetic position of C. fallax within the Brassicaceae family, we used the 66 PCGs from 29 Brassicaceae cp genomes and two outgroup cp genomes. The phylogenetic analysis was performed based on the maximum likelihood (ML) method and the GTRGAMMA model using RAxML v8.2.X with 1000 bootstrap replications (Stamatakis Citation2014). Phylogenetic tree analysis demonstrated that all Cardamine species formed a monophyletic clade, and C. fallax was a close relationship with the sister clade C. amaraeformis and C. parviflora (). The C. fallax cp genome could be used to distinguish C. parviflora and resolve the phylogenetic relationships within the Cardamine lineage.
Figure 1. Phylogenetic tree showing the relationship between Cardamine fallax and 30 Brassicales species. The phylogenetic tree was constructed based on 66 protein-coding genes of chloroplast genomes using maximum likelihood (ML) with 1000 bootstrap replicates. Numbers in each node indicated the bootstrap support values.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The data that support the findings of this study are openly available in NCBI (https://www.ncbi.nlm.nih.gov) GenBank with the accession number MZ043778. The associated BioProject, SRA and Bio-Sample numbers are PRJNA738661, SRR14845143 and SAMN19736473, respectively.
Additional information
Funding
References
- Al-Shehbaz IA, Arai K, Ohba H. 2006. Cardamine. In: Iwatsuki K, Boufford DE, Ohba H, editors. Flora of Japan, vol IIa, Angiospermae, Dicotyledoneae, Archichlamydeae(a). Tokyo: Kodansha Ltd.; p. 482–490.
- Al-Shehbaz IA. 1988. The genera of Arabideae (Cruciferae, Brassicaceae) in the Southeastern United States. J Arnold Arbor. 69:85–166.
- Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120.
- Cheo TY, Guo RL, Lan YZ, Lou LL, Kuan KC, An ZX. 1987. Cruciferae. In: Cheo TY, editor. Flora Reipublicae Popularis Sinicae, vol 33. Beijing: Science; p. 1–483.
- Doyle J. 1990. Isolation of plant DNA from fresh tissue. Focus. 12:13–15.
- Greiner S, Lehwark P, Bock R. 2019. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 47(W1):W59–W64.
- Kimata M. 1983. Comparative studies on the reproductive systems of Cardamine flexuosa, C. impatiens, C. scutata, and C. lyrata, Cruciferae. Bot Mag Tokyo. 96(4):299–312.
- Kitagawa M. 1982. Cruciferae. In: Satake Y, Ohwi J, Kitamura S, Watari S, Tominari T, editors, Wild flowers of Japan. Herbaceous plants (including dwarf shrubs) II in Japanese. Tokyo: Heibonsha Ltd.; p. 127–138.
- Kudoh H, Ishiguri Y, Kawano S. 1993. Phenotypic variability in life history traits and phenology of field populations of Cardamine flexuosa and C. fallax (Cruciferae) in Honshu, Japan. Plant Species Biol. 8(1):7–20.
- Lee YN. 1996. Flora of Korea in Hangul. Seoul: Kyo-Hak Publishing Co. Ltd.
- Lihova J, Marhold K. 2006. Phylogenetic and diversity patterns in Cardamine (Brassicaceae) – a genus with conspicuous polyploid and reticulate evolution. In: Sharma AK, Sharma A, editors. Plant genome: biodiversity and evolution. Enfield: Science Publishers; p. 149–186.
- Marhold K, Lihová J, Al-Shehbaz IA, Kudoh H. 2007. The correct interpretation and lectotypification of the name Cardamine fallax (Brassicaceae)). J Plant Res. 120(5):655–660.
- Prjibelski A, Antipov D, Meleshko D, Lapidus A, Korobeynikov A. 2020. Using SPAdes de novo assembler. Curr Protoc Bioinformatics. 70(1):e102.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Zhou TY, Lu LL, Yang G, Al-Shehbaz IA. 2001. Brassicaceae. In: Wu ZY, Raven PH, editors, Flora of China, vol 8. Beijing/St Louis: Science/Missouri Botanical Garden Press; p. 1–193.
