Abstract
This study determined the mitochondrial genome (mitogenome) of Chanodichthys mongolicus from China's Qiantang River and analyzed its phylogenetic history in the Subfamily Cultrinae. Next-generation sequencing was used to obtain the mitogenome of C. mongolicus, GenBank Accession Number MZ032228. The mitochondrial genome length of C. mongolicus from China's Qiantang River is 16,622 bp. The genome contains 13 protein-coding genes, 22 transfer RNAs, two ribosomal RNAs, and two central noncoding regions (the control region and the origin of light strand replication). Based on BLAST comparisons, the sequence identity of C. mongolicus MZ032228 from China's Qiantang River was 99.84% to that of Ancherythroculter wangi MG783573 from China's Nei River, 99.75% to C. mongolicus AP009060 from Russia's Black River. The phylogenetic analysis is consistent with BLAST comparisons in confirming that A. wangi MG783573 and C. mongolicus MZ032228 show a high genetic similarity. This study also confirms mitochondrial DNA heteroplasmy in C. mongolicus for the first time and documents 35 heterogeneous loci that were detected.
Introduction
The Mongolian redfin Chanodichthys mongolicus (Basilewsky 1855) is an economically important species of ray-finned fish classified in the genus Chanodichthys. The species is widely distributed in many reservoirs and lakes of China (Chen Citation1998). The Mongolian redfin was once recognized as the main aquaculture species in China (Xie et al. Citation2019). However, due to overfishing, habitat destruction, and water pollution, C. mongolicus stocks in natural waters have seriously declined. In recent years, the artificial propagation and release of the Mongolian redfin have been carried out in some regions of China (Xie et al. Citation2019). At present, genetic studies on C. mongolicus are relatively few. Although some researchers have reported the mitochondrial genome (mitogenome) of C. mongolicus (Saitoh et al. Citation2006; Tong et al. Citation2014; Wei et al. Citation2016), comparative mitogenomic analyses of C. mongolicus from the different geographical populations have not been reported. In this study, the mitogenome of C. mongolicus from China's Qiantang River was determined for the first time. A comparative analysis of the mitogenomes among C. mongolicus was undertaken. Other culter fishes are also included in the analyses to confirm the phylogenetic position of C. mongolicus in the Cultrinae. In addition, mitochondrial DNA (mtDNA) heteroplasmy in C. mongolicus is investigated and documented for the first time.
Materials and methods
The Mongolian redfin was harvested from Qiantang River (120°10′13.15″E, 30°07′11.22″N) and deposited at the National Original Breeding Farm of black Amur bream from Qiantang River (120°07′21.99″E, 30°08′35.53″N, www.hznky.com, Kai Liu, [email protected]). The total genomic DNA from the fin tissue (assigned as MGB201906) was extracted by the phenol-chloroform extraction method (Green and Sambrook Citation2012). After the genomic DNA was quantified, the DNA was sonicated using a Covaris M220. The sheared DNA fragments were purified and used to construct a sequencing library with the following steps: end-polished, A-tailed, and ligated with the full-length adaptor for Illumina sequencing with further PCR amplification (Quail et al. Citation2008). Finally, the purified DNA fragments were subjected to next-generation sequencing (NGS). The NGS was performed by Personal Gene Technology CO., Ltd (Shanghai, China). The mitochondrial genome of the Mongolian redfin was obtained by sequence assembly on the NGS data using GetOrganelle ver. 1.7.3.5 (Jin et al. Citation2020) with average base coverage of 151.8. The accession was registered GenBank under accession number MZ032228. The annotation process was completed using the MITOFISH prediction server (Iwasaki et al. Citation2013). Mitogenome sequence consistency was compared by CGView Comparison Tool ver. 1.0 (Grant et al. Citation2012). Detection of mitochondrial heteroplasmy in C. mongolicus was performed by NOVOPlasty ver. 4.3.1 (Dierckxsens et al. Citation2020) and visualized by Circos ver. 0.69.8 (Krzywinski et al. Citation2009). The phylogenetic analysis was performed by IQ-TREE ver. 2.1.3 with the complete mitogenomes, under the TPM2 + F + I + G4 substitution model (Minh et al. Citation2020). Mitogenome sequences were firstly aligned using the MAFFT component of UGENE ver. 38.1 (Okonechnikov et al. Citation2012) and then trimmed using trimAl ver. 1.2rev57 (Capella-Gutierrez et al. Citation2009). SH-aLRT test values and Ultrafast Bootstrap values were given in percentages (Hoang et al. Citation2018).
Results and discussion
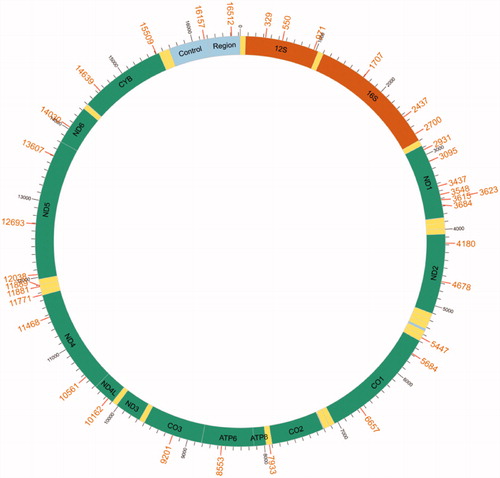
The mitogenome of C. mongolicus from China's Qiantang River contains 22 tRNAs, two ribosomal RNAs, 13 protein-coding genes (PCGs), and two central non-coding regions. Most genes of C. mongolicus are encoded on the heavy strand (H-strand) except for ND6 and eight tRNA, which are encoded on the light strand (L-strand). Within the genome, all 13 PCGs include the standard start codon ATG except for CO1, which is initiated with GTG. However, the stop codons of the 13 PCGs differ, these terminating with TAG, TAA, TA– or T––. The origin of light strand replication (OL), which extends up to 31 nucleotides, is identified in the WANCY region. The second non-coding region, the control region (D-loop), is 938 bp in length and located between tRNA-Pro and tRNA-Phe. The complete mitogenome of C. mongolicus from China's Qiantang River is 16,622 bp. The length is the same as two other C. mongolicus mitogenomes (Saitoh et al. Citation2006; Wei et al. Citation2016), and one bp longer than the mitogenome of C. mongolicus from China's Heilong River (Tong et al. Citation2014). Based on the comparison of BLAST results (), the sequence identity of C. mongolicus MZ032228 from China's Qiantang River is 99.84% to that of Ancherythroculter wangi MG783573 from China's Nei River (Zou et al. Citation2018), 99.75% to C. mongolicus AP009060 from Russia's Black River, 99.70% to C. mongolicus KF826087, 99.49% to C. mongolicus KC701385 from China's Heilong River, 97.18% to Ancherythroculter kurematsui KU234534 from China's Jialing River (Wang et al., Citation2016), and 97.17% to A. wangi MG575902 from China's Qi River (Yan et al. Citation2017).
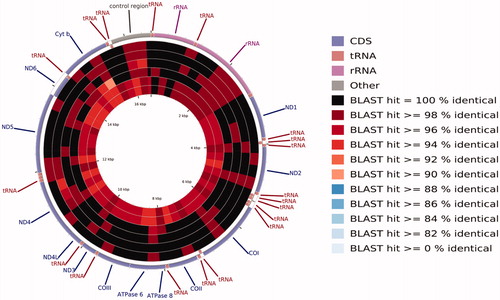
Figure 1. A BLAST-based pie chart of the sequence identity of the mitochondrial genomes of Chanodichthys mongolicus, Ancherythroculter wangi, and Ancherythroculter kurematsui from the different geographical populations. The circle from outside to inside represents Chanodichthys mongolicus MZ032228 from China's Qiantang River, Ancherythroculter wangi MG783573 from China's Nei River, Chanodichthys mongolicus AP009060 from Russia's Black River, Chanodichthys mongolicus KF826087, Chanodichthys mongolicus KC701385 from China's Heilong River, Ancherythroculter kurematsui KU234534 from China's Jialing River, and Ancherythroculter wangi MG575902 from China's Qi River
The phylogenetic analysis fully resolved C. mongolicus in a clade with four other published mitogenomes (). These included the three sequences of C. mongolicus cited above as well as the mitogenome of A. wangi MG783573. Within the clade, the SH-aLRT and Ultrafast Bootstrap values on some of the nodes were low. The A. wangi MG783573 sequence did not cluster with the other Ancherythroculter sequences, including another A. wangi MG575902 (Yan et al. Citation2017). This finding appears to point to an error or misidentification of that MG783573 specimen.
Figure 2. Phylogenetic tree of Chanodichthys mongolicus inferred using the maximum likelihood method based on the mitochondrial genome. Values are shown at each node of the tree, correspond to the SH-aLRT test values and Ultrafast Bootstrap value given in percentages.

mtDNA heteroplasmy in humans is common (Stewart and Chinnery Citation2015) and has been observed and studied in both invertebrates and vertebrates (Gajic et al. Citation2016; Gorkhali et al. Citation2016; Huang et al. Citation2019; Sriboonlert and Wonnapinij Citation2019). In C. mongolicus, 35 heterogeneous loci were detected with an average value of allele frequency and raw depth of 0.02 and 176.2, respectively (). The heterogeneous loci are mainly located in PCGs and are also present in the D-loop, some tRNAs, and rRNAs. Polymorphic sites in the PCGs resulted in 18 nonsynonymous and three synonymous substitutions. The ND1 gene showed the most heterogeneous loci, with seven loci. Based on the work by Wetjen et al. (Citation2017), the D-loop region was the primary region of mtDNA heterogeneity in fish detected by PCR methods. mtDNA heteroplasmy has also been identified in many gene locations in Loricarioidei using NGS data (Moreira et al. Citation2017). Heteroplasmic positions were found in almost all Loricarioidei species, except one. Mutations were scattered across the mitogenomes of Loricarioidei. The result of mtDNA heteroplasmy detected in Loricarioidei is similar to this study.
Disclosure statement
The authors reported no potential conflict of interest.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at [https://www.ncbi.nlm.nih.gov] (https://www.ncbi.nlm.nih.gov/) under the accession no. MZ032228. The associated BioProject, SRA, and BioSample numbers are PRJNA732476, SRX10977011, and SAMN19321491, respectively.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 25(15):1972–1973.
- Chen YY. 1998. Fauna Sinica: Osteichthyes Cypriniformes II. Beijing: Science Press.
- Dierckxsens N, Mardulyn P, Smits G. 2020. Unraveling heteroplasmy patterns with NOVOPlasty. NAR Genom Bioinform. 2(1):lqz011.
- Gajic B, Stevanovic J, Radulovic Z, Kulisic Z, Vejnovic B, Glavinic U, Stanimirovic Z. 2016. Haplotype identification and detection of mitochondrial DNA heteroplasmy in Varroa destructor mites using ARMS and PCR-RFLP methods. Exp Appl Acarol. 70:287–297.
- Gorkhali NA, Jiang L, Shrestha BS, He XH, Junzhao Q, Han JL, Ma YH. 2016. High occurrence of mitochondrial heteroplasmy in Nepalese indigenous sheep (Ovis aries) compared to Chinese sheep. Mitochondrial DNA Part A. 27(4):2645–2647.
- Grant JR, Arantes AS, Stothard P. 2012. Comparing thousands of circular genomes using the CGView Comparison Tool. BMC Genomics. 13:202.
- Green MR, Sambrook J. 2012. Molecular cloning: a laboratory manual, 4th ed. New York: Cold Spring Harbor Laboratory Press.
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 35(2):518–522.
- Huang Y, Lu W, Ji J, Zhang X, Zhang P, Chen W. 2019. Heteroplasmy in the complete chicken mitochondrial genome. PLOS One. 14(11):e0224677.
- Iwasaki W, Fukunaga T, Isagozawa R, Yamada K, Maeda Y, Satoh TP, Sado T, Mabuchi K, Takeshima H, Miya M, et al. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol Biol Evol. 30(11):2531–2540.
- Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, Li DZ. 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 21(1):241.
- Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res. 19(9):1639–1645.
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 37(5):1530–1534.
- Moreira DA, Buckup PA, Furtado C, Val AL, Schama R, Parente TE. 2017. Reducing the information gap on Loricarioidei (Siluriformes) mitochondrial genomics. BMC Genomics. 18(1):345.
- Okonechnikov K, Golosova O, Fursov M, UGENE team 2012. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics. 28(8):1166–1167.
- Quail MA, Kozarewa I, Smith F, Scally A, Stephens PJ, Durbin R, Swerdlow H, Turner DJ. 2008. A large genome center's improvements to the Illumina sequencing system. Nat Methods. 5(12):1005–1010.
- Saitoh K, Sado T, Mayden RL, Hanzawa N, Nakamura K, Nishida M, Miya M. 2006. Mitogenomic evolution and interrelationships of the Cypriniformes (Actinopterygii: Ostariophysi): the first evidence toward resolution of higher-level relationships of the world's largest freshwater fish clade based on 59 whole mitogenome sequences. J Mol Evol. 63(6):826–841.
- Sriboonlert A, Wonnapinij P. 2019. Comparative mitochondrial genome analysis of the firefly, Inflata indica (Coleoptera: Lampyridae) and the first evidence of heteroplasmy in fireflies. Int J Biol Macromol. 121:671–676.
- Stewart JB, Chinnery PF. 2015. The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat Rev Genet. 16(9):530–542.
- Tong GX, Kuang YY, Geng LW, Xu W, Yin JS. 2014. Mitochondrial DNA sequence of Mongolian redfin (Chanodichthys mongolicus). Mitochondrial DNA. 25(5):407–409.
- Wang GJ, Zheng ZL, Yu EM, Xie J, Wei N, Wu JR, Li JS. 2016. The complete mitochondrial genome of Ancherythroculter kurematsui (Cypriniformes: Cyprinidae). Mitochondrial DNA Part B. 1(1):630–631.
- Wei M, Yang Y, Yu P, Pan D, Wan Q. 2016. The complete mitochondrial genome of Mongolian redfin, Chanodichthys mongolicus (Cypriniformes: Cyprinidae): genome description and related phylogenetic analyses. Mitochondrial DNA Part A. 27(1):20–21.
- Wetjen M, Cortey M, Vera M, Schmidt T, Schulz R, García-Marín J-L. 2017. Occurrence of length polymorphism and heteroplasmy in brown trout. Gene Rep. 6:1–7.
- Xie JY, Yan Y, Yang YH, Lin SQ. 2019. Analysis on genetic structure of Chanodichthys mongolicus populations by mitochondrial COI gene sequences. Freshw Fish. 49(03):3–7.
- Yan T, Wang X, Zhang S, He L, He Z. 2017. The complete mitochondrial DNA of Ancherythroculter wangi. Mitochondrial DNA Part B. 3(1):19–20.
- Zou Y, Zhang J, Xie M, Zhang T, Deng Q, Wen Z. 2018. The complete mitochondrial genome of Ancherythroculter wangi and its phylogeny. Mitochondrial DNA Part B. 3(1):276–277.