Abstract
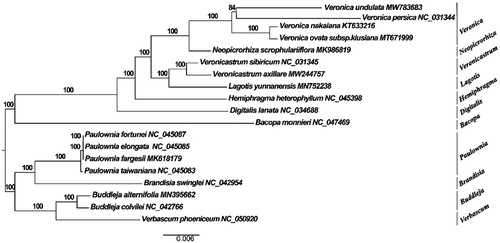
Veronica undulata is a perennial herb, and the complete chloroplast genome (plastome) of V. undulata was determined in this study. The results showed that the plastome size of V. undulata was 151,178 bp, including a large single-copy region (68,533 bp), a small single-copy region (21,403 bp), and two inverted repeat regions (25,566 bp). The total GC content of the plastome was 38.1%. We annotated 115 unique genes in the plastome, including 81 protein-coding genes (PCGs), 30 tRNAs, and four rRNAs. Phylogenetic analysis showed that the species of V. undulata and Veronica clustered together.
Keywords:
Veronica undulata is a perennial herb in the Plantaginaceae family (Albach and Chase Citation2001, Citation2004; Jensen et al. Citation2005). The species number in Veronica is very large, and many questions exist regarding the species classification of this genus (Albach et al. Citation2004). V. undulata was found on slopes flanking wetlands or ditches, which was widely distributed in China, Korea, Japan, Nepal, India, Pakistan, Afghanistan, Russian Federation, and the United States. As a large amphibious plant, V. undulata has important medicinal and ornamental value. Studies have shown that V. undulata was rich in iridoid glycosides (Taskova et al. Citation2010) and phenolic compounds (Li Citation1952; Boeger and Poulson Citation2003; Chen et al. Citation2003). V. undulata was previously reported to have cellular activity and anti-inflammatory activity (Saracoglu et al. Citation2011; Beara et al. Citation2015), which can help promote wound healing through hemostasis (Küpeli et al. Citation2005; Harput-Hudaverdi et al. Citation2008). In addition, the extraction of V. undulata also contains antioxidant, anticholinergic, and anticancer activities (Stojković et al. Citation2013; Sharifi-Rad et al. Citation2018). Therefore, V. undulata is considered a potential source of functional ingredients with a wide range of biological activities and holds great promise for pharmacological applications (Frontela-Saseta et al. Citation2013; He et al. Citation2015). In this study, the plastome of V. undulata was reported, which will provide a basic genetic resource for studying this important species and determining its phylogenetic position.
The fresh leaves of V. undulata were collected from the Lushan area of Shandong Province (36°21‘N, 118°4’E). The voucher specimens (20200709) of V. undulata were preserved at the College of Life Sciences, Shandong Normal University. The modified CTAB method was used for total plant DNA extraction (Doyle and Doyle Citation1987). Library preparation and paired-end sequencing work were completed on the Illumina Novaseq platform at Novogene (Beijing, China). Plastome was assembled with the Organelle Genome Assembler (OGA, https://github.com/quxiaojian/OGA). Plastome was annotated by the Plastid Genome Annotator (PGA, https://github.com/quxiaojian/PGA) (Qu et al. Citation2019) and manually corrected using Geneious v8.0.2 (Kearse et al. Citation2012). To further determine the phylogenetic position of V. undulata, the maximum likelihood (ML) tree was reconstructed using RAxML v8.2.10 with the 1000 rapid bootstrap replicates and the GTRGAMMA substitution model, using the alignment matrix of 115 unique genes generated by MAFFT v7.313 (Katoh and Standley Citation2013; Stamatakis Citation2014).
The complete plastome of V. undulata (GenBank accession number: MW783683) was 151,178 bp in length. The plastome was a quadripartite structure consisting of two single-copy regions separated by a pair of 21,539 bp inverted repeats. The large and small single-copy regions were 80,455 bp and 12,849 bp, respectively. The total GC content was 38.1%. A total of 115 unique genes were annotated in this plastome, including 81 protein-coding genes (PCGs), 30 tRNAs, and four rRNAs. Phylogenetic tree analysis showed that the species of V. undulata and Veronica clustered together ().
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession no. MW783683. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA720311, SRR14162363, and SAMN18646670, respectively.
Additional information
Funding
References
- Albach DC, Chase MW. 2001. Paraphyly of Veronica (Veroniceae; Scrophulariaceae): evidence from the internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA. J Plant Res. 114(1):9–18.
- Albach DC, Chase MW. 2004. Incongruence in Veroniceae (Plantaginaceae): evidence from two plastid and a nuclear ribosomal DNA region. Mol Phylogenet Evol. 32(1):183–197.
- Albach DC, Martínez–Ortega MM, Fischer MA, Chase MW. 2004. A new classification of the tribe Veroniceae – problems and a possible solution. Taxon. 53(2):429–452.
- Beara I, Živković J, Lesjak M, Ristić J, Šavikin K, Maksimović Z, Janković T. 2015. Phenolic profile and anti-inflammatory activity of three Veronica species. Ind Crop Prod. 63:276–280.
- Boeger MRT, Poulson ME. 2003. Morphological adaptations and photosynthetic rates of amphibious Veronica anagallis-aquatica L. (Scrophulariaceae) under different flow regimes. Aquat Bot. 75(2):123–135.
- Chen X-X, Lang F-Y, Xu Z-H, He J-H, Ma Y. 2003. The occurrence of leafminers and their parasitoids on vegetables and weeds in Hangzhou area, Southeast China. BioControl. 48(5):515–527.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin. 19(1):11–15.
- Frontela-Saseta C, Lopez-Nicolas R, Gonzalez-Bermudez CA, Martinez-Gracia C, Ros-Berruezo G. 2013. Anti-inflammatory properties of fruit juices enriched with pine bark extract in an in vitro model of inflamed human intestinal epithelium: the effect of gastrointestinal digestion. Food Chem Toxicol. 53:94–99.
- Harput-Hudaverdi US, Oztunca FH, Saracoglu I. 2008. Comparative phytochemical and biological studies on Veronica cuneifolia subsp. cuneifolia and V. cymbalaria. Planta Med. 74(09):PC:88.
- He M-X, Hu Q-C, Zhu Q-L, Pan K, Li Q. 2015. The feasibility of using constructed wetlands plants to produce bioethanol. Environ Prog Sustainable Energy. 34(1):276–281.
- Jensen SR, Albach DC, Ohno T, Grayer RJ. 2005. Veronica: Iridoids and cornoside as chemosystematic markers. Biochem Syst Ecol. 33(10):1031–1047.
- Katoh K, Standley DM. 2013. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol. 30(4):772–780.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649.
- Küpeli E, Harput US, Varel M, Yesilada E, Saracoglu I. 2005. Bioassay-guided isolation of iridoid glucosides with antinociceptive and anti-inflammatory activities from Veronica anagallis-aquatica L. J Ethnopharmacol. 102(2):170–176.
- Li H-L. 1952. The Genus Veronica (Scrophulariaceae) in China. Proc Acad Nat Sci Phila. 104:197–218.
- Qu X-J, Moore MJ, Li D-Z, Yi T-S. 2019. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 15(1):50.
- Saracoglu I, Oztunca FH, Nagatsu A, Harput US. 2011. Iridoid content and biological activities of Veronica cuneifolia subsp. cuneifolia and V. cymbalaria. Pharm Biol. 49(11):1150–1157.
- Sharifi-Rad J, Tayeboon GS, Niknam F, Sharifi-Rad M, Mohajeri M, Salehi B, Iriti M, Sharifi-Rad M. 2018. Veronica persica Poir. extract – antibacterial, antifungal and scolicidal activities, and inhibitory potential on acetylcholinesterase, tyrosinase, lipoxygenase and xanthine oxidase. Cell Mol Biol. 64(8):50–56.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Stojković DS, Živković J, Soković M, Glamočlija J, Ferreira ICFR, Janković T, Maksimović Z. 2013. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem Toxicol. 55:209–213.
- Taskova RM, Kokubun T, Ryan KG, Garnock-Jones PJ, Jensen SRJC, Bulletin P. 2010. Phenylethanoid and iridoid glycosides in the New Zealand snow hebes (Veronica, Plantaginaceae). Chem Pharm Bull. 58(5):703–711.