Abstract
We characterized the complete chloroplast genome of a perennial woody plant species, Salix linearistipularis, based on high-throughput sequencing and de novo assembly technology for the first time. The complete chloroplast genome of S. linearistipularis is 155,564 bp in length, comprising one large single-copy region (LSC, 84,460 bp), one small single-copy region (SSC, 16,182 bp), and two inverted repeat regions (IRA and IRB, 27,461 bp). The GC content of the whole chloroplast genome was 36.69%. This chloroplast genome encodes a total of 132 genes, including 86 protein-coding genes, eight ribosomal RNA genes, and 37 tRNA genes. Phylogenetic analysis reveals that S. linearistipularis is grouped with 13 other Salix species in Salicaceae.
Salix linearistipularis (syn. S. mongolica) is a shrub or small tree classified in the genus Salix of the Salicaceae family that is found in Inner Mongolia, Mongolia, three northeastern provinces, and (Far East) Russia. As the perennial woody species naturally distributed in the saline-alkali soil of the Songnen Plain in Northeast China (Ishida et al. Citation2009), S. linearistipularis exhibits strong endurance to salt stress and easy reproduction. Thus, it is broadly used for landscaping, alkali soil improvement, sand fixation, and reforestation with high ecological and economic benefits (Nan et al. Citation2016). Furthermore, the identification of genes for stress tolerance in this dioecious plant can facilitate the study of sex differentiation (a.Feng et al. Citation2020) and sex-related salt tolerance mechanisms (b.Feng et al. Citation2020). Nevertheless, due to the highly efficient crossing rate among Salix species, the classification of the genus Salix spp. is still disordered (Chen et al. Citation2010). Fortunately, the chloroplast genome of plants is conserved across evolution and has been widely and reliably used to evaluate relationships between closely related species. In this study, the complete chloroplast genome of S. linearistipularis was characterized for the first time to further investigate the genetic background of this species. The data will set a molecular foundation for the exploitation and conservation of willow resources.
Fresh leaves of S. linearistipularis were sampled from Yanchi County, Ningxia, China (37°47'N, 107°25'E) for DNA extraction. The extracted DNA was stored at −80 °C at the Key Laboratory of Forest Genetics and Biotechnology at Nanjing Forestry University. The voucher specimens were deposited in the herbarium of Nanjing Forestry University.
(https://www.njfu.edu.cn/, voucher number: NXHL2017003; Xiaoping Li, [email protected]). Whole genomic DNA was extracted from fresh leaves of S. linearistipularis by a modified CTAB method (Doyle and Doyle Citation1987) and fragmented to construct a 2 × 150 bp library for Illumina HiSeq sequencing (Illumina, San Diego, CA). After filtering the 5,574,054 raw reads, 4,848,768 high-quality reads were assembled by NOVOPlasty software
(https://github.com/ndierckx/NOVOPlasty) (Dierckxsens et al. Citation2017). The filtered scaffolds were aligned, oriented, and combined based on overlapping regions to construct the complete circular chloroplast genome sequence. The assembled chloroplast genome sequences of S. linearistipularis were uploaded to the online software GeSeq (https://chlorobox.mpimp-golm.mpg.de/) (Tillich et al. Citation2017) for preliminary annotation, and the initial annotation results were manually modified by comparison with the chloroplast genome of S. gordejevii (MW562004). The complete chloroplast genome sequence was submitted to GenBank under the accession number MZ018223. To determine the phylogenetic position of S. linearistipularis in Salicaceae, the chloroplast genome sequences of S. linearistipularis and 32 members of the Salicaceae family were aligned by MAFFT v.7.475 (Katoh and Standley Citation2013) (https://mafft.cbrc.jp/alignment/software/). Then, phylogenetic analysis based on the maximum-likelihood (ML) method was executed in MEGA X (Kumar et al. Citation2018) with 1000 bootstraps. Itoa orientalis in the family Flacourtiaceae served as the root.
The whole chloroplast genome of S. linearistipularis is 155,564 bp in length and exhibits a typical quadripartite structure comprising one large single-copy region (LSC, 84,460 bp), one small single-copy region (SSC, 16,182 bp), and two inverted repeat regions (IRA and IRB, 27,461 bp). The GC content of the whole plastid genome was 36.69%, and the respective contents of the LSC, SSC, and IR regions were 34.41%, 31.03%, and 41.86%. The chloroplast genome of S. linearistipularis encoded a total of 132 genes, including 86 protein-coding genes, eight rRNA genes, and 37 tRNA genes. The ycf1 gene is located at the IRa/SSC border as a pseudogene. One rps12 gene is split into two individual transcripts. While the majority of genes are present in single-copy form, four rRNA genes, seven tRNA genes, and eight protein-coding genes have two copies. A total of 18 genes contained introns, three of which (rps12, ycf3, clpP) included two introns, and the remaining 15 genes contained only one intron.
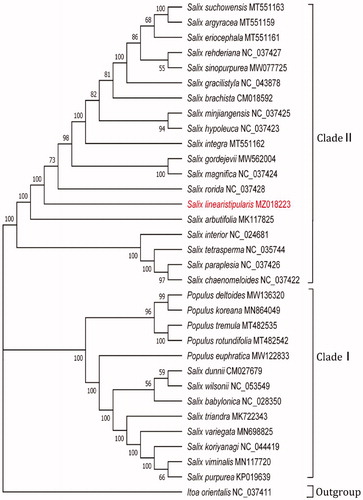
The results of phylogenetic analyses yielded further evidence of the phylogenetic taxonomy in Salicaceae. As illustrated in , two major clades were identified with strong support values (BP = 100%). Clade I contained two subclades: Subclade I (BP = 100%) and Subclade II (BP = 96%). Within Clade II, 19 species in Salix were separated into two subclades (BP = 100%). The first subclade included S. interior, S. tetrasperma, S. paraplesia and S. chaenomeloides. Within the second subclade, S. arbutifolia was placed as the basal species and clustered with the other 14 species in Salix (BP = 100%). Here, S. linearistipularis was grouped with 13 other Salix species in Salicaceae (S. suchowensis, S. argyracea, S. eriocephala, S. rehderiana, S. sinopurpurea, S. gracilistyla, S. brachista, S. minjiangensis, S. hypoleuca, S. integra, S. gordejevii, S. magnifica, S. rorida) (BP = 73%).
Geolocation information
Nanjing Forestry University, 159 Longpan Road, Xuanwu District, Nanjing City, Jiangsu Province
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/nuccore/MZ018223.1/, reference number MZ018223. The raw sequence data used in this research were deposited successfully with registered numbers of associated BioProject, SRA, and Bio-Sample: PRJNA734150, SRR14700532, and SAMN19473694, respectively.
Additional information
Funding
References
- Chen JH, Hang S, Wen J, Yang YP. 2010. Molecular phylogeny of Salix L.(Salicaceae) inferred from three chloroplast datasets and is systematic impliations. Taxon. 59(1):29- 37.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVO plasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11- 15.
- a.Feng S, Ren LL, Sun HW, Qiao K, Liu SK, Zhou AM. 2020. Morphological and physiological responses of two willow species from different habitats to salt stress. Sci Rep. 10(1):18228.
- b.Feng S, Ren LL, Sun HW, Ma HP, Zhang XP, Ma SR, Qiao K, Zhou AM, Bu YY, Liu SK. 2020. Sexual differences in physiological and transcriptional responses to salinity stress of Salix linearistipularis. Front Plant Sci. 11(11):517962.
- Ishida TA, Nara K, Ma SR, Takano T, Liu SK. 2009. Ectomycorhizal fungal community in alkaline-saline soil in northeastern China. Mycorhiza. 19(5):329- 335.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772- 780.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547- 1549.
- Nan GX, Zhang Y, Li S, Imshik L, Tetsuo T, Liu SK. 2016. NaCl stress-induced transcriptomics analysis of Salix linearistipularis (syn. Salix mongolica). J Biol Res-Thessaloniki. 23(1):1–14.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6- W11.