Abstract
The complete plastome of Amaranthus retroflexus L., a field weed, was identified in this study. The genome size was 150,710 bp and consists of a large single-copy (LSC: 83,892 bp) region, a small single-copy (SSC: 18,100 bp) region, and two inverted repeats (IRs: 24,359 bp) regions. GC content was 36.6%. A total of 113 genes were identified, including 79 protein-coding genes, four rRNA genes, and 30 tRNA genes. Twenty chloroplast genomes from Amaranthaceae were selected to reconstruct phylogenetic tree and the result supported that A. retroflexus was sister to A. hypochondriacus and A. caudatus.
Keywords:
Amaranthus retroflexus L. is a monoecious annual herb within Amaranthaceae, widely distributed all over the world. A. retroflexus is native to North America, it is dramatically expanded its distribution throughout the China for the past decades (Weber et al. Citation2008). This weed is a prolific seed producer and seriously endangers the growth of crops in farmland (Francischini et al. Citation2014). The A. retroflexus increasingly develop resistance due to extensively use of herbicides (Robertson Citation1985; Li et al. Citation2004; Powles and Yu Citation2010). This study reported the A. retroflexus complete plastome, which would provide a fundamental genetic resources for weeds prevention and analyzing its phylogenetic position.
Fresh leaves of A. retroflexus were collected from Changdao District (Shandong, China; 37°91′ N, 120°73′ E). A specimen was deposited at Herbarium of College of Life Sciences, Shandong Normal University (Shou-Jin Fan, Email: [email protected]) under the voucher number 20120. Total genomic DNA was extracted using a modified CTAB method (Zhang et al. Citation2019; Guo et al. Citation2020). The library preparation and paired-end (PE) sequencing of total genomic DNA were conducted by the Illumina Novaseq platform at Novogene (Beijing, China). Organelle Genome Assembler (OGA, https://github.com/quxiaojian/OGA) was used to do plastome assembling. Annotation was accomplished with Plastid Genome Annotator (PGA, https://github.com/quxiaojian/PGA) (Qu et al. Citation2019). Manual annotation correction was performed through Geneious v9.1.4 (Kearse et al. Citation2012). In order to determine the phylogenetic position of A. retroflexus, a maximum-likelihood (ML) tree was reconstructed by RAxML v8.2.10 (Stamatakis Citation2014) using 1000 bootstrap replicates with GTRCAT model based on 73 protein-coding genes after alignment using MAFFT v7.313 (Katoh and Standley Citation2013).
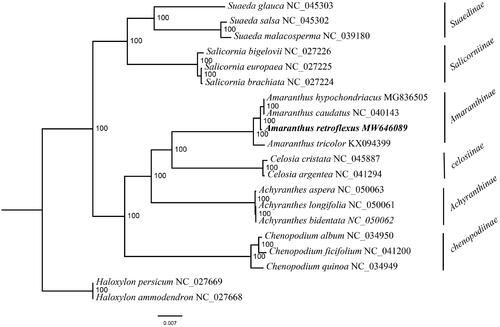
The complete plastome of A. retroflexus (GenBank accession number: MW646089) was 150,710 bp in length, and comprised a large single-copy (83,892 bp) region, a small single-copy (18,100 bp) region, and a pair of inverted repeats (IRs, 24,359 bp) regions. The GC content of this plastome was 36.6%. The GC content of IR regions is 42.6%, higher than LSC (34.5%) and SSC (30.2%) regions. A total of 113 unique genes were encoded, including 79 PCGs, 30 tRNAs, and four rRNAs. There are genes with two copies, including ndhB, rpl2, rpl23, rps12, rps7, rrn16, rrn23, rrn4.5, rrn5, trnA-UGC, trnI-CAU, trnL-CAA, trnN-GUU, trnR-ACG, trnV-GAC, and ycf2. The ML phylogenetic tree showed that A. retroflexus was sister to A. hypochondriacus and A. caudatus (). In conclusion, the plastome of A. retroflexus provides significant DNA molecular data for further phylogenetic and evolutionary analysis for Amaranthus.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are openly available in GenBank of NCBI, reference number MW646089. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA718096 (http://www.ncbi.nlm.nih.gov/bioproject/718096), SRR14089437 (https://www.ncbi.nlm.nih.gov/sra/PRJNA718096), and SAMN18522283 (https://www.ncbi.nlm.nih.gov/sra/PRJNA718096), respectively.
Additional information
Funding
References
- Francischini AC, Constantin J, Oliveira RS Jr, Santos G, Franchini LHM, Biffe DF. 2014. Resistencia de Amaranthus retroflexus a herbicidas inibidores da enzima acetolactato sintase. Planta Daninha. 32(2):437–446.
- Guo XX, Dai C, Wang R, Qu XJ, Zhang XJ. 2020. Characterization and phylogenetic analysis of the complete plastome of Alopecurus japonicus (Gramineae), an annual weed. Mitochondrial DNA Part B. 5(1):396–397.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649.
- Li XX, Zhang HJ, Ni HW. 2004. Review on the biological characters and control of redroot pigweed (Amaranthus retroflexus). Pesticide Sci Admin. 25(3):13–16.
- Powles SB, Yu Q. 2010. Evolution in action: plants resistant to herbicides. Annu Rev Plant Biol. 61(1):317–347.
- Qu XJ, Moore MJ, Li DZ, Yi TS. 2019. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 15(1):1–12.
- Robertson D. 1985. Origin and phenotypic expression of recessive chloroplast mutations in higher plants: atrazine resistance as a model system. Plant Mol Biol Rep. 3(3):99–106.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Weber E, Sun SG, Li B. 2008. Invasive alien plants in China: diversity and ecological insights. Biol Invasions. 10(8):1411–1429.
- Zhang XJ, Wang N, Zhang LY, Fan SJ, Qu XJ. 2019. Characterization of the complete plastome of Atriplex centralasiatica (Chenopodiaceae), an annual halophytic herb. Mitochondrial DNA Part B. 4(2):2475–2476.