Abstract
Epimedium L. is the largest herbaceous genus in the family Berberidaceae which comprises more than 60 species. Epimedium sutchuenense Franch. is narrowly inhabited in the Daba Mountains of China. In the current study, we assembled the first complete chloroplast genome of E. sutchuenense through Illumina paired-end sequencing. The complete chloroplast genome of E. sutchuenense was 157,218 bp in length and the total GC content was 38.78%. A total of 112 unique genes were identified, including 78 protein-coding genes, 30 tRNA genes and 4 rRNA genes. The phylogenetic analysis demonstrated that E. sutchuenense was sister to Epimedium wushanense T. S. Ying. Our results provided valuable information for further phylogenetic research and germplasm exploration of Epimedium genus.
Epimedium L. is the largest herbaceous genus belonging to the family Berberidaceae with more than 60 perennial plant species discontinuously distributed from North Africa (Algeria) to East Asia (Stearn Citation2002; Ying Citation2002). Since more than 50 Epimedium species had been discovered in China, covering more than 80 percent of this genus, China is now considered to be the modern diversity center of Epimedium species (De Smet et al. Citation2012). The leaves of Epimedium plants had long been used as traditional Chinese medicine ‘Herba Epimedii’ for their special effects of nourishing kidney, muscles, and bones. Prenylated flavonol glycosides (such as Icariin, epimedii A, B, and C), as the main components of Herba Epimedii, has been verified to possess wide-reaching bioactive activities such as regulating bone modeling, anti-tumor, anti-aging, etc. (Liu et al. Citation2006; Wu et al. Citation2003; Ma et al. Citation2011; Yang et al. Citation2019).
However, the infrageneric classification of Epimedium genus remains debatable due to frequent interspecific hybridization and gene introgression. In modern phylogenetic research, chloroplast genomes have been extensively used due to their special advantages such as moderate nucleotide substitution rate, relatively conserved gene sequence and genome structure (Zhang and Li Citation2011). Therefore, it is still necessary to sequence and assemble chloroplast genomes from more species in order to clarify the intractable phylogenetic relationships within Epimedium genus.
In 1884, Franchet (French botanist) published Epimedium fargesii Franch. and Epimedium sutchuenense Franch. based on the type specimen that Paul Farges (a French missionary) collected in the Chengkou county of Chongqing city (Franchet Citation1886). E. sutchuenense is narrowly distributed in the Daba Mountains (mainly in the Wanyuan County and Qu County of Sichuan province, the Wuxi County, Chengkou County, and Kai County of Chongqing city, and the Shennongjia Forestry District in Hubei province, China) and it is used as “Herba Epimedii” by local people. Specially, E. sutchuenense is unique among Epimedium species for its long-creeping rhizome and the narrowly lanceolate inner sepals which are about as long as the petals (Stearn, Citation2002). Furthermore, controversies existed all along about whether E. sutchuenense should be used as ‘Herba Epimedii’ since prenylated flavonoid and its glycosides are nearly absent in E. sutchuenense (Guo and Xiao Citation1996; Qin et al. Citation2020). In this study, we report the first complete chloroplast genome of E. sutchuenense and the results will provide useful data for resolving the phylogenetic relationships within Epimedium genus.
For this study, the E. sutchuenense was sampled from the Shennongjia Forestry District in Hubei province, China (latitude 31.7467 and longitude 110.6709). A specimen and the extracted DNA were deposited at Medicinal Plants Authentication Center, Institute of Medicinal Plant Development, Chinese Academy of Medical Science, Beijing, China (http://www.implad.ac.cn/, collected by Baolin Guo, [email protected]) under the voucher number B. L. Guo 0437. Genomic DNA was extracted from the fresh leaves of E. sutchuenense with the modified CTAB method (Doyle and Doyle Citation1987). The high-quality DNA was sheared to an average size of 300 bp for library construction using the VAHTSTM Universal DNA Library Pren Kit (ExCell Bio. Biological Technology Co., Ltd, Shanghai, China), and then was sequenced on the Illumina Novaseq 6000 platform (Illumina Inc., San Diego, CA). For assembly, GetOrganelle v1.5 (Jin et al. Citation2018) was employed to assemble the full length of chloroplast genome sequences with E. acuminatum (GenBank accession number: NC_029941) as reference. The annotation of chloroplast genome was conducted through the online program CPGAVAS2 (Shi et al. Citation2019) and followed by manual correction. The annotated genomic sequence was registered into GenBank with an accession number (MW483087).
The complete chloroplast genome of E. sutchuenense was 157,218 bp in length, including two inverted repeat regions (IRA and IRB, 25,782 bp) separated by a large single copy region (LSC, 88,575 bp) and a small single copy region (SSC, 17,079 bp). The total GC content was 38.78%, with IR regions having the highest GC content (43.20%), followed by the LSC (37.38%) and SSC region (32.77%). A total of 112 unique genes were identified from the chloroplast genome of E. sutchuenense, including 78 protein-coding genes, 30 tRNA genes and 4 rRNA genes. The intron-exon structure analysis showed that a total of 18 genes were found to have introns, among which petB, petD, rpl16, rpl2, rpoC1, rps16, trnA-UGC, trnG-UCC, trnI-GAU, trnK-UUU, trnL-UAA, trnV-UAC, atpF, ndhA and ndhB have one intron, while ycf3, rps12, and clpP contain two introns.
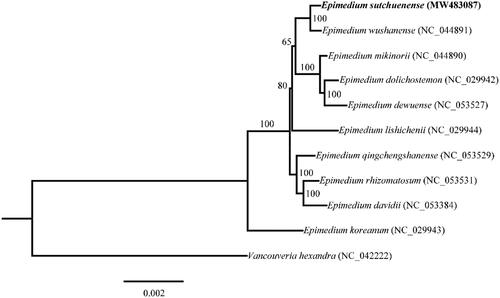
To determine the phylogenetic position of E. sutchuenense, phylogenetic analysis was performed using the complete chloroplast genome sequences of E. sutchuenense and other 10 species downloaded from the NCBI GenBank database. Multiple sequence alignments were generated by using MAFFT v7 (Katoh et al. Citation2019) and then a Maximum Likelihood (ML) tree was constructed by using IQ-TREE multicore v 2.0.3 (Minh et al. Citation2020) with Vancouveria hexandra (Hook.) C. Morren & Decne as the outgroup (). The phylogenetic analysis revealed that E. sutchuenense formed a sister relationship with Epimedium wushanense T. S. Ying. Our results provided useful information for future research on the evolutionary relationships within the Epimedium genus.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession no. MW483087. The associated numbers are PRJN751491, SRR15323871, and SAMN20520045, respectively.
Additional information
Funding
References
- De Smet Y, Goetghebeur P, Wanke S, Asselman P, Samain MS. 2012. Additional evidence for recent divergence of Chinese Epimedium (Berberidaceae) derived from AFLP, chloroplast and nuclear data supplemented with characterization of leaflet pubescence. Plecevo. 145(1):73–87.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Franchet AM. 1886. Sur les especes du genre Epimedium. Bull Soc Bot France. 33(1):38–41.
- Guo B, Xiao P. 1996. Prospects on the quality evaluation and resource utilization of medicinal plants from genus Epimedium L. Nat Prod Res Dev. 8(01):74–78.
- Jin J, Yu W, Yang J, Song Y, Yi T, Li D. 2018. GetOrganelle: a simple and fast pipeline for de novo assembly of a complete circular chloroplast genome using genome skimming data. BioRxiv. 4:256479.
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 20(4):1160–1166.
- Liu JJ, Li SP, Wang YT. 2006. Optimization for quantitative determination of four flavonoids in Epimedium by capillary zone electrophoresis coupled with diode array detection using central composite design. J Chromatogr A. 1103(2):344–349.
- Ma H, He X, Yang Y, Li M, Hao D, Jia Z. 2011. The genus Epimedium: an ethnopharmacological and phytochemical review. J Ethnopharmacol. 134(3):519–541.
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 37(5):1530–1534.
- Qin W, Wei Y, Yang Y, Liu X. 2020. Study on the correlation between inflorescence characteristics and flavonoid content of large-flowered taxa of Epimedium. Nat Prod Res Dev. 32(04):633–644.
- Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, Liu C. 2019. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 47(W1):W65–W73.
- Stearn WT. 2002. The genus Epimedium and other herbaceous Berberidaceae. Portland (OR): Timber Press.
- Wu H, Lien EJ, Lien LL. 2003. Chemical and pharmacological investigations of Epimedium species: A survey. In: Jucker E, editor. Progress in drug research. Basel: Birkhäuser; p. 1–57.
- Yang Q, Pan J, Shen G, Guo B. 2019. Yellow light promotes the growth and accumulation of bioactive flavonoids in Epimedium pseudowushanense. J Photochem Photobiol B. 197:111550.
- Ying T. 2002. Petal evolution and distribution patterns of Epimedium L. (Berberidaceae). Acta Phytotaxon Sin. 40(6):481–489.
- Zhang Y, Li D. 2011. Advances in phylogenomics based on complete chloroplast genomes. Plant Diversity Resour. 33(4):365–375.