Abstract
The emergence of weedy rice (Oryza sativa f. spontanea) has been considered as a serious global agricultural problem in recent decades. To better understand its speciation, here we assembled the complete chloroplast genome of O. sativa f. spontanea with the length of 134,502 bp. The assembly contains a large single-copy (LSC, 80,549 bp), a small single-copy (SSC, 12,347 bp) and a pair of inverted repeats (IRa and IRb, 20,803 bp each). A total of 132 unique genes were annotated, including 82 protein-coding genes, 42 tRNA genes and eight rRNA genes. Phylogenetic analysis showed that O. sativa f. spontanea (indica type) appears closely related to cultivated indica rice rather than wild rice, supporting the hypothesis that weedy rice originated from cultivated rice.
Weedy rice (Oryza sativa f. spontanea) refers to the unwanted Oryza plants in paddy fields that appear with some undesired agronomic traits, such as high degrees of seed shattering and seed dormancy, dramatically distinguished from cultivated rice (Qiu et al. Citation2020). At present, weedy rice has become one of the most common and noxious weeds in global paddy fields and has caused serious agricultural problems, as it could reduce the crop yields by greater than 80% without artificial weeding (Estorninos et al. Citation2005; Oerke Citation2006; Ziska et al. Citation2015). As its genomic background is similar to cultivated rice, it is difficult to eliminate weedy rice by applying regular herbicides (Nadir et al. Citation2017). Chloroplast genomes provide useful information to species identification and evolutionary studies. No chloroplast genomes of O. sativa f. spontanea have been available. Thus, here we assembled the complete chloroplast genome of O. sativa f. spontanea (indica type).
The sample of O. sativa f. spontanea (indica type) used in this study was collected in Changxing County, Zhejiang Province, China (30°55′17.7″N, 119°51′58.9″E) and deposited in the Herbarium of Zhejiang University (HZU, http://sweetgum.nybg.org/science/ih) with the accession number HZU60244003. The whole-genome high-throughput sequencing data was generated throught our previous study (Qiu et al. Citation2020). The raw reads were first filtered into clean data using NGSQCtoolkit v2.3 (Patel and Jain Citation2012). Using the cultivated indica rice (Oryza sativa) complete chloroplast genome (GenBank accession number NC_031333.1) as a reference, NOVOPlasty v3.6 (Dierckxsens et al. Citation2017) was used in the de novo assembly. Genome annotation was performed by the GeSeq online (Tillich et al. Citation2017). The assembled genome sequences and annotation information have been deposited in GenBank under the accession number LC642244.
The total length of O. sativa f. spontanea (indica type) chloroplast genome is 134,502 bp, which displays the typical quadripartite structure of most angiosperm chloroplast genomes, including the large single-copy (LSC, 80,549 bp), the small single-copy (SSC, 12,347 bp) and a pair of inverted repeats (IRa and IRb, 20,803 bp each). The GC contents of the LSC and SSC, IR regions are 37.1%, 33.3% and 44.3%, respectively. A total of 132 unique genes were annotated, including 82 protein-coding genes, 42 tRNA genes and eight rRNA genes. 21 genes were duplicated in the IR regions.
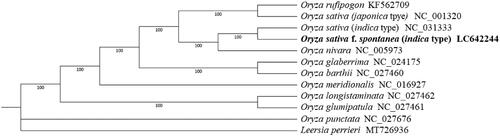
To help clarify understand the phylogenetic relationship between O. sativa f. spontanea (indica type) and other Oryza species, the chloroplast genome sequences of ten Oryza species and Leersia perrieri were downloaded from GenBank and used for phylogeny construction. We first performed sequence alignment using MAFFT v7.310 (Katoh et al. Citation2002) with the parameter ‘–auto –reorder –phylipout.’ IQ-tree v1.6.12 was then used to construct the phylogenetic tree using L. perrieri as an outgroup with the recommended parameter ‘-m MFP -bb 1000 -bnni’ (Nguyen et al. Citation2015). Finally, the tree was illustrated and modified by iTOL (Letunic and Bork Citation2019). The phylogenetic result showed that O. sativa f. spontanea (indica type) was genetically close to cultivated rice O. sativa (indica type) rather than O. nivara or other wild rice (), which supported the hypothesis that O. sativa f. spontanea originated from cultivated rice (Qiu et al. Citation2017).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data of this study is available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov) under the accession number LC642244. The associated BioProject, SRA, and BioSample numbers are PRJNA606132, SRX7710350, and SAMN14085936 respectively.
Additional information
Funding
References
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18–e18.
- Estorninos LE, Gealy DR, Gbur EE, Talbert RE, McClelland MR. 2005. Rice and red rice interference. II. Rice response to population densities of three red rice (Oryza sativa) ecotypes. Weed Sci. 53(5):683–689.
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30(14):3059–3066.
- Letunic I, Bork P. 2019. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47(W1):W256–59.
- Nadir S, Xiong HB, Zhu Q, Zhang XL, Xu HY, et al. 2017. Weedy rice in sustainable rice production. A review. Agron Sustain Dev. 37(5):46.
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.
- Oerke EC. 2006. Crop losses to pests. J Agric Sci. 144(1):31–43.
- Patel RK, Jain M. 2012. NGS QC toolkit: a toolkit for quality control of next generation sequencing data. PLoS One. 7(2):e30619.
- Qiu J, Jia L, Wu D, Weng X, Chen L, Sun J, Chen M, Mao L, Jiang B, Ye C, et al. 2020. Diverse genetic mechanisms underlie worldwide convergent rice feralization. Genome Biol. 21(1):70.
- Qiu J, Zhou Y, Mao L, Ye C, Wang W, Zhang J, Yu Y, Fu F, Wang Y, Qian F, et al. 2017. Genomic variation associated with local adaptation of weedy rice during de-domestication. Nat Commun. 8(1):15323.
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. 2017. GeSeq – versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 45(W1):W6–11.
- Ziska LH, Gealy DR, Burgos N, Caicedo AL, Gressel J, et al. 2015. Chapter Three–- Weedy (Red) rice: an emerging constraint to global rice production. In: Sparks DL editor, Advances in agronomy, Vol. 129. Cambridge: Academic Press; p. 181–228.