Abstract
The complete mitochondrial genome of Tylonycteris fulvida (Peters, 1872) was obtained using high-throughput sequencing technology. The genome is a circular molecule of 16,621 bp length, containing 13 protein-coding genes, 2 rRNA genes, 22 tRNA genes, and a control region. A phylogenetic tree of 13 protein-coding genes was constructed using IQ-TREE. Our result suggests that T. fulvida cluster within Chiroptera and Fereuungulata. The complete mitochondrial genome sequence of T. fulvida will be helpful for future taxonomic and phylogenetic studies on Chiroptera.
The lesser bamboo bat (Tylonycteris fulvida) is belonging to Laurasiatheria, Chiroptera, Vespertilionidae, which are the first family including most speciose in bats (Amador et al. Citation2018). The previous phylogenetic study suggested two popular hypotheses among Laurasiatheria, i.e. the ‘Fereuungulata’ and the ‘Pegasoferae’ hypothesis (Murphy et al. Citation2007; dos Reis et al. Citation2012; Foley et al. Citation2016; Esselstyn et al. Citation2017). These hypotheses are related to which order (Chiroptera or Cetartiodactyla) is the seconding-branching laurasiatherian lineage. In this study, we generated a complete mitogenome of T. fulvida (GenkBank accession: MZ457524) from China which maybe contributes to our understanding of the phylogenetic relationship within Laurasiatheria.
An adult female of T. fulvida (Voucher No. GZHU17245) was collected Guangdong Province, China (23°17′25.9″N, 113°24′35.8″E). The liver tissue was kept in 95% ethanol at −20 °C laboratory freezer in Guangzhou University (CONTACT: Wenhua Yu, email: [email protected]). Complete genomic DNA was extracted from MiniBEST Universal Genomic DNA Extraction Kit (TAKARA, Dalian) and was sequenced using MGISEQ-2000RS with a PE150 protocol. Following the MitoZ tutorial (Meng et al. Citation2019), a total of 5 G base pairs (bp) were obtained, then a complete mitochondrial genome was further generated and automatically annotated.
The complete mitogenome of T. fulvida is 16,621 bp long with a base composition of 12.75% G, 34.48% A, 30.37% T, and 22.40% C. It encoded 37 genes including 13 protein-coding genes, 22 tRNA genes, 2 rRNA genes, and a control region (D-loop). All these genes were encoded on the heavy strand, except for the ND6 protein-coding gene and eight tRNA genes (tRNAGln, tRNAAla, tRNAAsn, tRNACys, tRNATyr, tRNASer, tRNAGlu, and tRNAPro) which were located on light strand. The total length of protein-coding genes was 11,436 bp, occupying 68.80% of the total length. All protein initiation codons are ATG, except for ND2, ND5 with ATA, and ND3 with ATT. Nine protein-coding genes (ND1, COX1, COX2, ATP8, ATP6, ND4L, ND5, ND6, Cyt b) terminate with the stop codon TAA, while the ND2 and ND3 end with TAG. Besides, incomplete stop codon (T-- or TA-) was observed in ND4 and COX3, respectively.
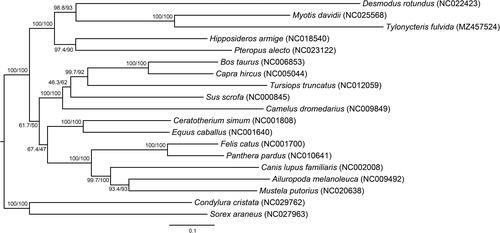
To verify the phylogenetic hypothesis, the 13 protein-coding genes of 19 laurasiatherian species were used to reconstruct the phylogenetic tree that was rooted by Condylura cristata and Sorex araneus (). All the sequences were aligned using MAFFT (Katoh and Standley Citation2013) and subsequently cleaned using Gblocks (Castresana Citation2000). Next, the best model for each protein-coding gene was selected using ModelFinder (Kalyaanamoorthy et al. Citation2017) as implemented in IQ-TREE v. 1.6.12 (Nguyen et al. Citation2015). Following the model selection, the best ML (maximum likelihood) tree was estimated with 1000 ultrafast bootstraps (UFboot) (option: -bb 1000) (Hoang et al. Citation2018) and the SH-like approximate likelihood ratio test (SH-aLRT) (option: -alrt 1000) (Guindon et al. Citation2010) using IQ-TREE. The ML tree showed that the Chiroptera was the seconding-branching laurasiatherian lineage with 100% bootstrap support which supported the ‘Fereuungulata’ hypothesis. Mitochondrial genome of T. fulvida could benefit future phylogenetic and evolutionary studies on Laurasiatheria and Chiroptera.
Acknowledgments
We are particularly grateful to Xiaoyun Wang, Fang Li, Yifeng Hu and Yannan Li for their contribution to collecting sample and data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are openly available in NCBI at https://www.ncbi.nlm.nih.gov/nuccore/ MZ457524, reference number MZ457524. The associated BioProject, SRA and Bio-Sample numbers are PRJNA741550, SRR15195148 and SAMN19884333, respectively.
Additional information
Funding
References
- Amador LI, Arévalo RLM, Almeida FC, Catalano SA, Giannini NP. 2018. Bat systematics in the light of unconstrained analyses of a comprehensive molecular supermatrix. J Mammal Evol. 25(1):37–70.
- Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 17(4):540–552.
- dos Reis M, Inoue J, Hasegawa M, Asher RJ, Donoghue PC, Yang Z. 2012. Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proc Biol Sci. 279(1742):3491–3500.
- Esselstyn JA, Oliveros CH, Swanson MT, Faircloth BC. 2017. Investigating difficult nodes in the placental mammal tree with expanded taxon sampling and thousands of ultraconserved elements. Genome Biol Evol. 9(9):2308–2321.
- Foley NM, Springer MS, Teeling EC. 2016. Mammal madness: is the mammal tree of life not yet resolved? Phil Trans R Soc B. 371(1699):20150140.
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59(3):307–321.
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 35(2):518–522.
- Kalyaanamoorthy S, Minh BQ, Wong TKF, Von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–589.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software Version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Meng G, Li Y, Yang C, Liu S. 2019. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 47(11):e63–e63.
- Murphy WJ, Pringle TH, Crider TA, Springer MS, Miller W. 2007. Using genomic data to unravel the root of the placental mammal phylogeny. Genome Res. 17(4):413–421.
- Nguyen L-T, Schmidt HA, Von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274.