Abstract
Arisaema heterophyllum Blume is a perennial medicinal herb widely distributed in China, Korea and Japan. In this study, the complete chloroplast genome sequence of A. heterophyllum was assembled and characterized based on high-throughput sequencing data. The whole chloroplast genome is 170,610 bp in length and contains 95,485 bp large single-copy (LSC) and 22,605 bp small single-copy (SSC) regions separated by a pair of 26,260 bp inverted repeat (IR) regions. It contained a total of 129 genes, including 84 protein-coding genes, 37 tRNA genes, and 8 rRNA genes, with an overall GC content of 34.5%. A phylogenetic tree reconstructed by 30 chloroplast genomes reveals that A. heterophyllum is mostly related to the same genus A. ringens, A. franchetianum and A. erubescens. The complete chloroplast genome of A. heterophyllum was the firstly reported and deposited at GenBank under accession number MZ424448.
Arisaema heterophyllum Blume is a perennial herbaceous medicinal plant of the Arisaema genus of Araceae and widely distributed in China, Korea and Japan (Wang et al. Citation2018). Its dried tuber is a traditional Chinese medicine with a long history usage, which was named Arisaema and listed in Chinese Pharmacopeia with the function of dissipating binds and dispersing swelling (Wang et al. Citation2012). Pharmacological analysis indicated that A. heterophyllum possessed many pharmacological activities, mainly including anti-tumor (Feng et al. Citation2016), anti-bacterial (Wang et al. Citation2004), analgesic (Ye et al. Citation2010) and anti-infammatory (Wang et al. Citation2012). Alkaloids, favonoids, plant lectins, lignans and terpenes are its main medicinal ingredients (Yang et al. Citation2013; Kant et al. Citation2020). However, its phylogenetic position is not very clear causing on the lack of genomic information. Here, we characterized the complete chloroplast genome sequence of A. heterophyllum according to high throughput sequencing technology, which will provide a powerful informatics data for the phylogeny of A. heterophyllum and other related species.
The fresh leaves of A. heterophyllum were collected from Lu’an, Anhui, China (31°77′N, 115°93′E). Specimens were stored in the Herbarium of West Anhui University (https://hsx.wxc.edu.cn/, Shanyong Yi with the email [email protected]) under the voucher number WAU-YYTNX-20210413-1. Total genomic DNA was extracted from the leaves material according to a modified CTAB protocol (Doyle and Doyle Citation1987). The DNA was stored at −80 °C in our lab. The whole genome sequencing was conducted by Hefei Biodata Biotechnologies Inc. (Hefei, China) on the Illumina Hiseq platform. The filtered sequences were assembled using the program SPAdes assembler 3.10.0 (Bankevich et al. Citation2012). The DOGMA (Wyman et al. Citation2004) and BLAST searches were employed for the annotation.
The chloroplast genome of A. heterophyllum was determined to comprise a 170,610 bp double stranded, circular DNA (GenBank accession no. MZ424448), which containing two inverted repeat (IR) regions of 26,260 bp, separated by large single-copy (LSC) and small single-copy (SSC) regions of 95,485 bp and 22,605 bp, respectively. The genome was predicted to have 129 genes, including 84 protein-coding genes, 37 tRNA genes, and 8 rRNA genes. Five protein-coding genes, seven tRNA genes and four rRNA genes were duplicated in IR regions. Nineteen genes contained two exons and four genes (clpP, ycf3 and two rps12) contained three exons. The overall GC content of A. heterophyllum cp genome is 34.5% and the corresponding values in LSC, SSC and IR regions are 32.3%, 27.9% and 41.3%, respectively.
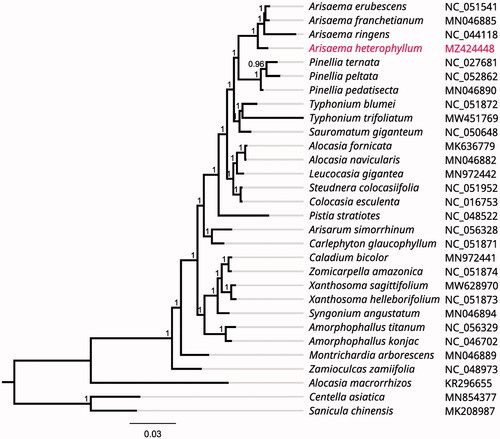
To investigate its taxonomic status, alignment was performed with 30 reported chloroplast genome (full DNA) sequences (Centella asiatica and Sanicula chinensis were used as outgroup) using MAFFT v7.307 (Katoh and Standley Citation2013), and a maximum likelihood (ML) tree was produced by FastTree version 2.1.10 (Price Citation2010). As expected, A. heterophyllum is mostly related to the same genus A. ringens, A. franchetianum and A. erubescens with bootstrap support values of 100% (). In addition, the results also showed that the species from the genus Arisae ma (A. heterophyllum, A. ringens, A. franchetianum and A. erubescens) were closer to those from the genus Pinellia (Pinellia ternata, P. peltata and P. pedatisecta) in the genetic relationship among the family Araceae.
Figure 1. Phylogenetic tree inferred by Maximum Likelihood (ML) method based on 30 representative species. Centella asiatica and Sanicula chinensis were used as outgroup. A total of 1000 bootstrap replicates were computed and the bootstrap support values are shown at the branches. GenBank accession numbers were shown in .
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data of A. heterophyllum that support the findings of this study are openly available in GenBank of NCBI at (https://www.ncbi.nlm.nih.gov/) under the accession no. MZ424448. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA743035, SRR15014599, and SAMN19989665, respectively.
Additional information
Funding
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. 2012. SPAdes: a new genomeassembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19(5):455–477.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15.
- Feng LX, Sun P, Mi T, Liu M, Liu W, Yao S, Cao YM, Yu XL, Wu WY, Jiang BH, Yang M, et al. 2016. Agglutinin isolated from Arisema heterophyllum Blume induces apoptosis and autophagy in A549 cells through inhibiting PI3K/Akt pathway and inducing ER stress. Chin J Nat Med. 14(11):856–864.
- Kant K, Lal UR, Rawat R, Kumar A, Ghosh M. 2020. Genus Arisaema: a review of traditional importance, chemistry and biological activities. Comb Chem High Throughput Screen. 23(7):624–648.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780.
- Price MN, Dehal PS, Arkin AP. 2010. FastTree 2–approximately maximum-likelihood trees for large alignments. PloS One. 5(3):e9490.
- Wang C, Zhu J, Liu M, Yang Q, Wu J, Li Z. 2018. De novo sequencing and transcriptome assembly of Arisaema heterophyllum Blume and identification of genes involved in isoflavonoid biosynthesis. Sci Rep. 8(1):17643.
- Wang G, Jiang D, Fang H. 2004. Study on bacteriostatic action and mechanism of Arisaema consanguineum Schott. Acta Veterinaria et Zootechnica Sinica. 3:280–285.
- Wang LW, Xu BG, Wang JY, Su ZZ, Lin FC, Zhang CL, Kubicek CP. 2012. Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens. Appl Microbiol Biotechnol. 93(3):1231–1239.
- Wyman SK, Jansen RK, Boore JL. 2004. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 20(17):3252–3255.
- Yang LI, Qian LU, Qian J, University D. 2013. Study on anti-inflammatory effect and mechanism of the extract in Arisaema erubescens. J Dali Univ. 12(9):14–16.
- Ye M, Sun DZ, Qin ZF. 2010. Clinical observation of Xiaotan Tongluo Gel for external application in the treatment of cancer pain. Chin J Inform Tradit Chin Med. 17(7):22–24.
