Abstract
The greater round-eared bat, Tonatia bidens, is a locally rare species belonging to the highly diverse family Phyllostomidae. In this study, the complete mitogenome of T. bidens was sequenced using optimized protocols of DNA extraction from fixed cells originally prepared for cytogenetic studies. Here we present the complete mitogenome and place our results in a phylogenetic context with other data generated for the family Phyllostomidae. The circular genome had 16,717 bp in size, comprising 37 genes and GC content of 42.24%. Furthermore, the phylogenetic tree indicated a well-supported relationship between the representatives of Tonatia into the subfamily Phyllostominae.
Round-eared bats of the genus Tonatia (Phyllostomidae, Phyllostominae) are widely distributed throughout Latin America and represented by three extant species: Tonatia bidens Spix 1823, T. bakeri and T. maresi Williams, Willig & Reid 1995, the last two recently elevated from subspecies of T. saurophila to species rank (Basantes et al. Citation2020). Despite their wide distribution, little information encompassing aspects of their biology is known, and most authors consider the representatives of Tonatia as rare species due to few occurrences in nature, generally in small groups, especially T. bidens (Willig Citation1985; Esbérard and Bergallo Citation2004).
Tonatia bidens is a medium-sized bat, endemic to South America found in Argentina, Paraguay, Bolivia, and Brazil. Scarce incidence with fragmented and punctual sampling data justifies its categorization as Data Deficient (Barquez and Diaz Citation2016). Therefore, by describing the mitogenome of T. bidens, we provide new insights into extranuclear genome evolution as well as initial steps into the genetic characterization for this species. Herein, one male specimen of T. bidens was collected in Parque Estadual Pedra da Boca (6° 27′ 32.2″S, 35° 40′ 45.4″ W), Araruna, Paraíba, Brazil, during field expeditions in 2004, as authorized by IBAMA (Licence no. 12264-1 to Santos, N). The voucher specimen was deposited in the mammalian collection of Departamento de Sistemática e Ecologia at Universidade Federal da Paraiba, João Pessoa, Brazil (www.ccen.ufpb.br/museubiologia/) under the voucher number UFPB5719.
Total genomic DNA was extracted from fixed cytological suspension (stored in methanol-acetic acid, 3:1, v/v) and deposited in the Laboratório de Genética e Citogenética Animal e Humana (LGCAH/UFPE; Voucher ID: M829). Fixed cells in suspension were washed twice with 1 × PBS (Life Technologies, pH 7.4) for 20 min before DNA extraction using the DNeasy Blood & Tissue kit (Qiagen), following the manufacturer’s instructions for blood samples. Paired-end libraries were built with Nextera DNA Flex Library Prep kit (Illumina) and sequenced using a high-output v2 kit (300 cycles) on an Illumina NextSeq 500 platform. The mitogenome was assembled using NovoPlasty 3.6 (Dierckxsens et al. Citation2017) and annotation was performed with MITOS2 (Bernt et al. Citation2013), with minor manual corrections in Geneious Prime 2020.2 (Biomatters).
The mitogenome of T. bidens (GenBank accession MZ391834) comprised 16,717 bp, with two ribosomal RNA (rrnL and rrnS) genes, 13 protein-coding genes (PCGs) and 22 transfer RNA (tRNA). The nucleotide composition was 32.3% A, 29.1% C, 13.1% G, and 25.4% T, with 42.24% of GC content. The average size and gene arrangement were identical to those of other bat species (Meganathan et al. Citation2012; Botero-Castro et al. Citation2013, Citation2018). In addition, two rRNA and most of tRNA and PCGs were encoded in the H-strand, whereas NAD6 was in the L-strand, besides eight tRNAs (trnA, trnC, trnE, trnN, trnP. trnQ, trnS2[tga] and trnY). Tonatia bidens had 10 ORFs starting with ATG, two starting with ATA (NAD5 and NAD2), and one starting with ATT (NAD3).
We investigated the relationships among T. bidens and other 20 phyllostomid species using available mitogenomes recovered from GenBank, and assigning the sequenced mitogenomes of representatives of the families Mormoopidae, Mystacinidae, and Noctilionidae as outgroups. The phylogenetic analyses were based on all PCGs amino acid (AA) sequences aligned with MAFFT 7.3 (Katoh et al. Citation2002). Maximum likelihood (ML) trees were obtained with RAxML v8.2 (Stamatakis Citation2014) using the substitution model PROTGAMMA and rapid bootstraping (BS) with 1,000 replicates ().
Figure 1. Maximum likelihood showing the relationship among subfamilies of phyllostomid bats and position of Tonatia bidens based on amino acid sequences of 13 PGCs of the mitochondrial genomes. The families Mormoopidae, Noctilionidae, and Mystacinidae are used as outgroups. The respective GenBank accession numbers are shown. Bootstrap values are evidenced near the nodes of the clades. * The mitogenome of former T. saurophila (Botero-Castro et al. Citation2013), now T. maresi, based on sampling locality (French Guiana; Basantes et al. Citation2020).
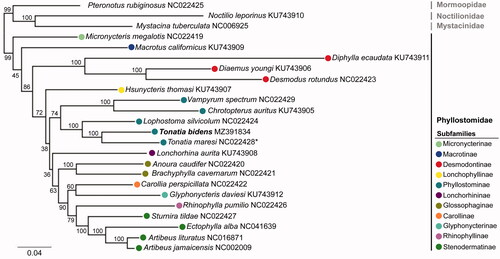
The phylogenetic analysis recovered a monophyletic Phyllostomidae clade with strong support (BS = 99) and included mitogenomes from representatives of all eleven subfamilies from the classification proposed by Baker et al. (Citation2003, Citation2016; ). Our analysis resulted mostly in well-supported clades (BS >70). In addition, in contrast with the Baker et al. (Citation2003, Citation2016) phylogenies, incongruences were observed in the positions of the subfamilies Micronycterinae relative to Macrotinae, Lonchophyllinae in alternative branching relative to other nectarivorous bats, and Lonchorhininae appeared as a lineage that diverged after Phyllostominae, most of these relations resulted in low-supported clades (), although a similar mitogenome AA-tree was observed by Botero-Castro et al. (Citation2018). Within the subfamily Phyllostominae (BS = 74), T. bidens was recovered as sister to T. maresi (BS = 100), and the genus Tonatia was more closely related to Lophostoma than to Chrotopterus and Vampyrum (), as already observed in other phylogenetic approaches (Baker et al. Citation2003; Rojas et al. Citation2016; Basantes et al. Citation2020). The present study serves as encouragement to other cytogenetic laboratories to take advantage of cytological material for new purposes, especially in rare species, as provided here useful knowledge of genomic data in this species which will be crucial for the understanding biodiversity and future studies in the taxonomy and molecular systematics of phyllostomid bats.
Ethics statement
All procedures performed in this study involving animals followed guidelines established by Animal Care and Use guidelines of ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade), and using the collecting permit number (12264-1) by the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA).
Author contribution statement
JCF, SV, and CGS-C contributed to the conception and design of the study, analysis, and interpretation of the data. JCF wrote the first version of the manuscript. SV, GO, NS and CGS-C critically reviewed the article regarding its intellectual content. NS collected biological samples. All authors read, discussed, and approved the final version and all authors agree to be accountable for all aspects of the work.
Disclosure statement
The authors report no conflicts of interest.
Data availability statement
The mitogenome sequence data that support the finding of this study are openly available in GenBank of NCBI at [https://www.ncbi.nlm.nih.gov], under the accession no. MZ391834. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA785884, SRR17118535, and SAMN23588143, respectively.
Additional information
Funding
References
- Baker RJ, Hoofer SR, Porter CA, Van Den Bussche RA. 2003. Diversification among new world leaf-nosed bats: an evolutionary hypothesis and classification inferred from digenomic congruence of DNA sequence. Lubbock, TX: Occas Pap Mus Texas Tech Univ. 230:p. 1–32.
- Baker RJ, Solari S, Cirranello A, Simmons NB. 2016. Higher level classification of phyllostomid bats with a summary of DNA synapomorphies. Acta Chiropterol. 18(1):1–38.
- Barquez R, Diaz M. 2016. Tonatia bidens. The IUCN Red List of Threatened Species. 2016. e.T21983A21975435. [accessed 2021 June 03]. https://doi.org/https://dx.doi.org/10.2305/IUCN.UK.2016-2.RLTS.T21983A21975435.en
- Basantes M, Tinoco N, Velazco PM, Hofmann MJ, Rodríguez-Posada ME, Camacho MA. 2020. Systematics and taxonomy of Tonatia saurophila Koopman & Williams, 1951 (Chiroptera, Phyllostomidae). Zookeys. 915:59–86.
- Bernt M, Donath A, Juhling F, Externbrink F, Florentz C, Fritzsch G, Pütz J, Middendorf M, Stadler PF. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Mol Phylogenet Evol. 69(2):313–319.
- Botero-Castro F, Tilak M, Justy F, Catzeflis F, Delsuc F, Douzery EJP. 2018. In cold blood: compositional bias and positive selection drive the high evolutionary rate of vampire bats mitochondrial genomes. Genome Biol Evol. 10(9):2218–2239.
- Botero-Castro F, Tilak M, Justy F, Catzeflis F, Delsuc F, Douzery EJP. 2013. Next-generation sequencing and phylogenetic signal of complete mitochondrial genomes for resolving the evolutionary history of leaf-nosed bats (Phyllostomidae). Mol Phylogenet Evol. 69(3):728–739.
- Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18.
- Esbérard CEL, Bergallo HG. 2004. Aspectos sobre a biologia de Tonatia bidens (Spix) no estado do Rio de Janeiro, sudeste do Brasil (Mammalia, Chiroptera, Phyllostomidae). Rev Bras Zool. 21(2):253–259.
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30(14):3059–3066.
- Meganathan PR, Pagan HJT, MCCulloch ES, Stevens RD, Ray DA. 2012. Complete mitochondrial genome sequences of three bats species and whole genome mitochondrial analyses reveal patterns of codon bias and lend support to a basal split in Chiroptera. Gene. 492(1):121–129.
- Rojas D, Warsi OM, Dávalos LM. 2016. Bats (Chiroptera: Noctilionoidea) challenge a recent origin of extant Neotropical diversity. Syst Biol. 65(3):432–448.
- Stamatakis A. 2014. RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313.
- Willig MR. 1985. Reproductive patterns of bats from Caatingas and Cerrado Biomes in northeast Brazil. J Mammal. 66(4):668–681.
