Abstract
In the study, we report the complete mitochondrial genome of Artemia persimilis Piccinelli and Prosdocimi, 1968 for the first time. The mitochondrial genome of A. persimilis is 15,436 bp in length, with the typical structure of 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs) and 2 ribosomal RNA genes, and a non-coding control region (CR). Phylogenetic analysis showed that A. persimilis was at the basal position among the bisexual Artemia species, which revealed that A. persimilis is likely to be an ancestral clade. The present study could provide effective resources for population genetics study, as well as germplasm conservation in Artemia.
Introduction
Artemia is not only one of the most important live food items used in larval aquaculture, but also an ideal laboratory model organism for scientific research. As a small crustacean, Artemia have a widely distribution all over the world, mainly in hypersaline environments, and play an important biological regulatory role in salt field ecosystem. The genus Artemia is generally considered to contain seven bisexual species as well as some parthenogenetic Artemia populations with different polyploidy types (Asem et al. Citation2010). However, their generic taxonomy is not universally accepted, especially in relation to Artemia tibetiana and Artemia urmiana. Mitochondrial DNA are widely used to study the molecular ecology of animals because it is convenient and economical, comparison of the mitochondrial genomes will permit examination of evolution and relationships between species. At present the complete mitochondrial genome of four bisexual species (Artemia franciscana, A. urmiana, A. tibetiana and Artemia sinica) is already known (Valverde et al. Citation1994; Zhang et al. Citation2013; Asem et al. Citation2019). But for another bisexual species Artemia persimilis Piccinelli and Prosdocimi, 1968, a species endemic to the South American and geographically restricted to Argentina and Chile (Sainz Escudero et al. Citation2021), the complete mitochondrial genome has not been reported and characterized. Herein, we reported the complete mitochondrial genome sequence of A. persimilis, and we performed a phylogenetic analysis to study the evolutionary relationships of A. persimilis with other Artemia species. We expect that the present result will facilitate the further investigations of phylogenetic relationship, taxonomic resolution and phylogeography of the Artemia species.
Materials and methods
The cysts of A. persimilis Piccinelli and Prosdocimi, 1968 was collected from Bahia Blanca, Argentina (**latitude** −62.1338 and **longitude** −38.8385). The specimen was deposited at the Asian Regional Artemia Reference Center (Tianjin University of Science and Technology, China) (Liying Sui, [email protected]) under the voucher number 1807. The genomic DNA was extracted form cysts according to the instructions of TIANGEN®TIANamp Genomic DNA Kit (Tianjin, China). Then the high quality gDNA was sequenced using Illumina Novaseq6000 platform with 350 bp insert size. The complete mitochondrial genome was assembled using SPAdes v.3.5.0 (http://cab.spbu.ru/software/spades/) with A. franciscana (GenBank accession number: X69067) as reference. The reference mitochondrial map and BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) were used for gene annotation. The tRNA genes were predicted using the ARWEN (http://mbio-serv2.mbioekol.lu.se/ARWEN/) and tRNAscan-SE 2.0 (http://lowelab.ucsc.edu/tRNAscan-SE/) online software.
Results and discussion
The complete mitochondrial genome of A. persimilis Piccinelli and Prosdocimi, 1968 (Genbank assession number: MZ199176) is 15,436 bp in length, with the typical structure of 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs) and 2 ribosomal RNA genes, and a non-coding control region (CR). The base composition is 25.70% A, 24.00% C, 20.07% G, and 30.23% T, with an A + T content of 55.93%. Just six PCGs (cox1, atp6, cox3, cytb, nd1 and nd2) began with the common ATG start codon. Stop codons included TAA (cox3, nd2, atp8, atp6, nd3 and cytb) and TAG (nd1, nd4l and nd6). There are 4 genes ended with the incomplete stop codon T (cox1, cox2, nd4 and nd5). The 12S rRNA and 16S rRNA were separated by the trnV. The length of rrnL and rrnS is 1148 bp and 711 bp, respectively. The 22 tRNA genes size varies from 61 to 67 bp, respectively.
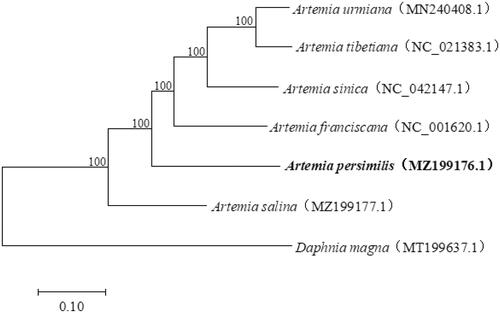
Combined with the complete mitochondrial genome sequences of four bisexual Artemia species as well as diecious Artemia salina from GenBank, a phylogenetic tree was constructed by Maximum-likelihood (ML) method with the Kimura 2-parameter model using the software MEGA 7.0 (Kumar et al. Citation2016), so as to infer the phylogenetic relationships among Artemia species. The phylogenetic tree showed that A. persimilis was at the basal position among the bisexual Artemia species, which revealed that A. persimilis is likely to be an ancestral clade (). The mitochondrial genome sequence of A. persimilis reported here provide a useful genetic resource for population genetics and evolutionary studies in Artemia.
Ethics statement
The study protocol was approved by the Committee on the Ethics of Animal Experiments of Tianjin University of Science and Technology.
Author contributions statement
Chi Zhang supervised the project. Xuekai Han and Tashi Lahm conceived and designed the study. Liying Sui and Guishuang Wang guided data analysis and interpretation. Xuekai Han performed experiments and drafted the manuscript. GUSANG Deji revised it critically for intellectual content. All authors approved the final version of the manuscript.
Acknowledgements
The authors thank Artemia Reference Center (Ghent University, Belgium) for assistance with specimen collection.
This study was funded by the Projects of Agricultural intelligence introduction of Tibet (2020WZ006) and the National Science Foundation of Tianjin (18JCQNJC78500).
Disclosure statement
The authors declare that they have no conflict of interest.
Data availability statement
The data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov, reference number MZ199176. The associated BioProject, SRA, and BioSample numbers are PRJNA749897, SRR15292985, and SAMN20424866, respectively.
Additional information
Funding
References
- Asem A, Li WD, Pei-Zheng Wang PZ, Eimanifar A, Shen CY, De Vos S, Van Stappen G. 2019. The complete mitochondrial genome of Artemiasinica Cai, 1989 (Crustacea: Anostraca) using next-generation sequencing. Mitochondrial DNA Part B. 4(1):746–747.
- Asem A, Rastegar-Pouyani N, De los Rios P. 2010. The genus Artemia Leach, 1819 (Crustacea: Branchiopoda): true and false taxonomical descriptions. Lat Am J Aquat Res. 38:501–506.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Bio Evol. 33(7):1870–1874.
- Sainz Escudero L, López Estrada EK, Rodríguez Flores PC, García París M. 2021. Settling taxonomic and nomenclatural problems in brine shrimps, Artemia (Crustacea: Branchiopoda: Anostraca), by integrating mitogenomics, marker discordances and nomenclature rules. Peer J. 9:e10865.
- Valverde J, Batuecas B, Moratilla C, Marco R, Garesse R. 1994. The complete mitochondrial DNA sequence of the Crustacean Artemia franciscana. J Mol Evol. 39(4):400–408.
- Zhang HX, Luo QB, Sun J, Liu F, Wu G, Yu J, Wang WW. 2013. Mitochondrial genome sequences of Artemia tibetiana and Artemia urmiana: assessing molecular changes for high plateau adaptation. Sci China Life Sci. 56(5):440–452.